Содержание
- 3. What are alcohols? Alcohols are a homologous series of organic compounds with the general formula CnH2n+1OH
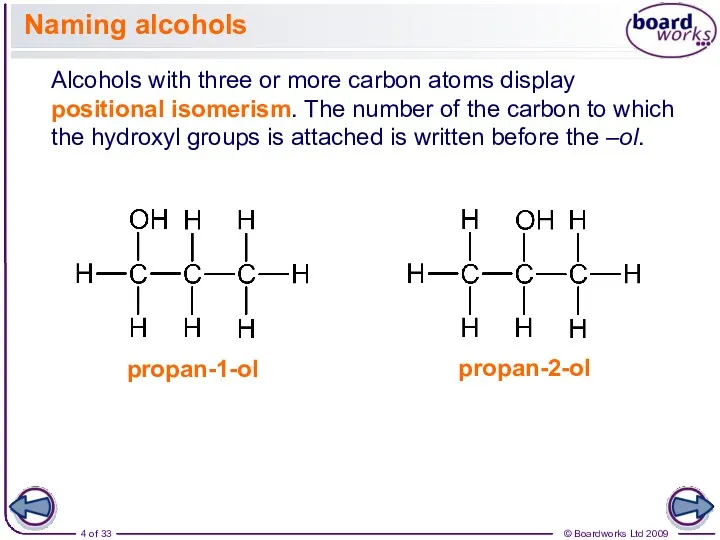
- 4. Naming alcohols Alcohols with three or more carbon atoms display positional isomerism. The number of the
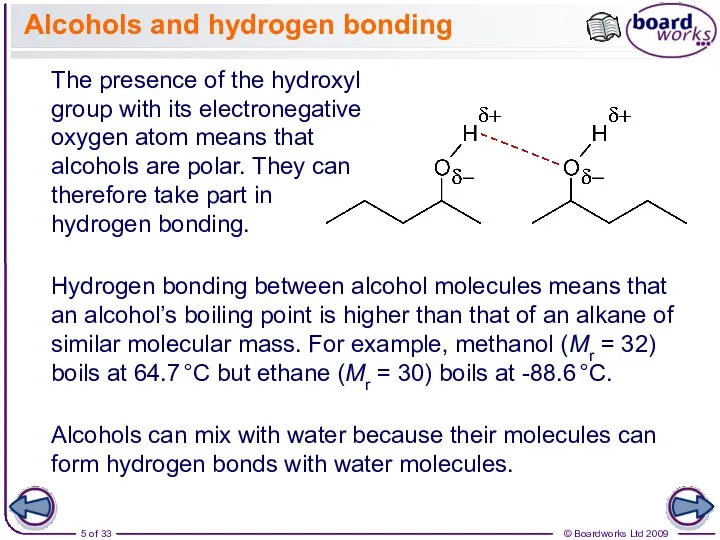
- 5. Alcohols and hydrogen bonding The presence of the hydroxyl group with its electronegative oxygen atom means
- 6. Making wine and cider Alcohol has been produced by fermentation of sugars for thousands of years.
- 7. Industrial fermentation Industrially, sugar cane, molasses (a product of refining sugar cane) or starch (from potatoes
- 8. Production of ethanol from ethene
- 9. Fermentation vs. hydration
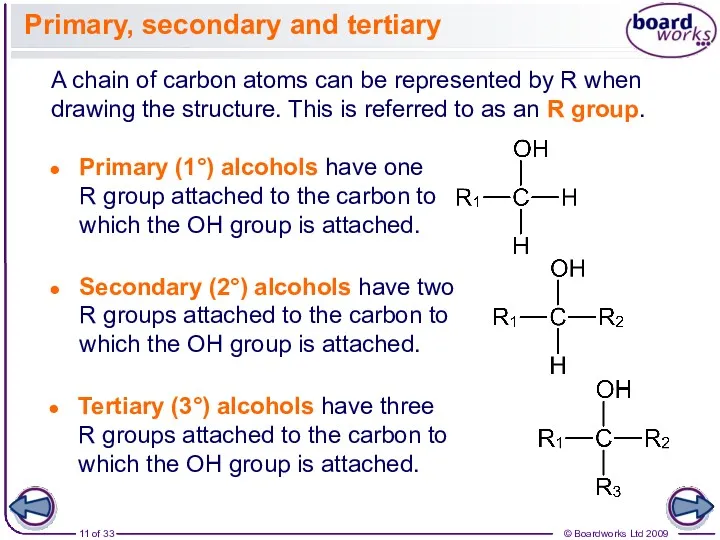
- 11. A chain of carbon atoms can be represented by R when drawing the structure. This is
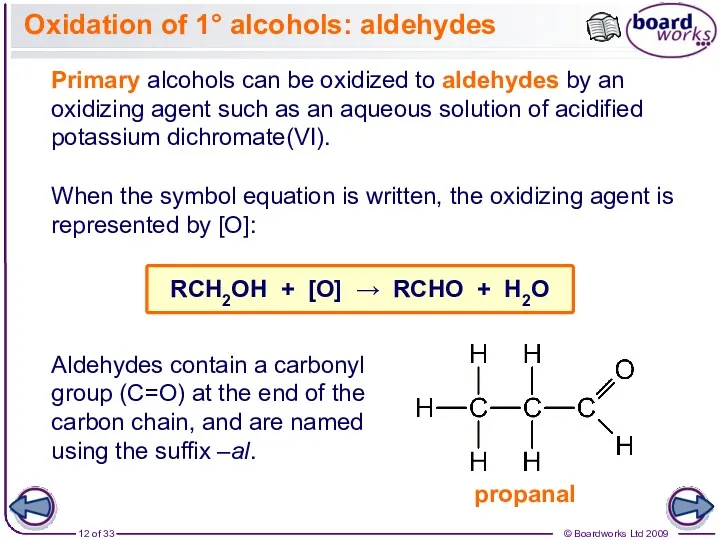
- 12. Oxidation of 1° alcohols: aldehydes Primary alcohols can be oxidized to aldehydes by an oxidizing agent
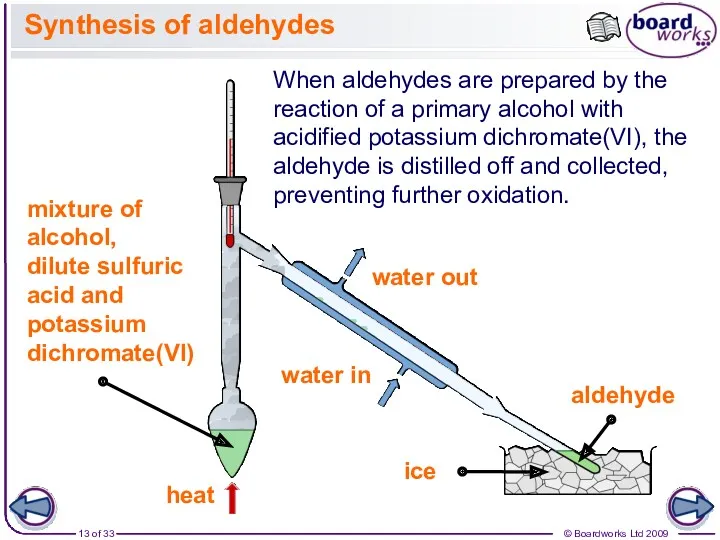
- 13. Synthesis of aldehydes When aldehydes are prepared by the reaction of a primary alcohol with acidified
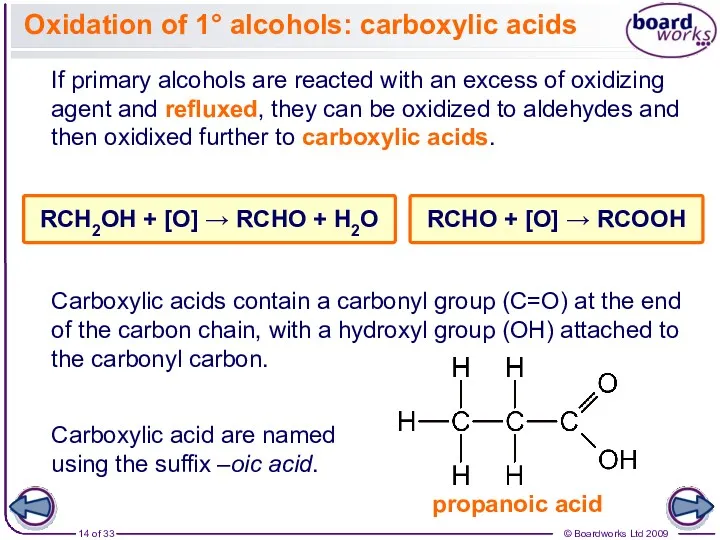
- 14. Oxidation of 1° alcohols: carboxylic acids If primary alcohols are reacted with an excess of oxidizing
- 15. Synthesis of carboxylic acids
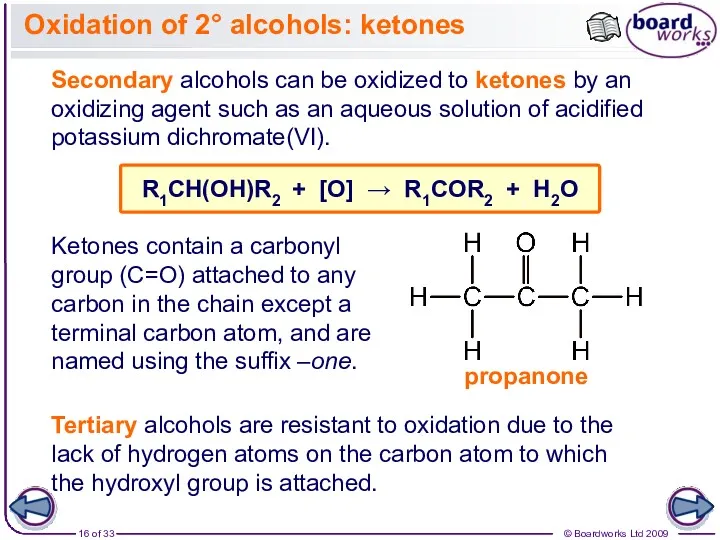
- 16. Oxidation of 2° alcohols: ketones Secondary alcohols can be oxidized to ketones by an oxidizing agent
- 17. Aldehyde, ketone or carboxylic acid?
- 18. Distinguishing aldehydes and ketones
- 19. Esterification Esterification involves refluxing a carboxylic acid and an alcohol with a concentrated sulfuric acid catalyst.
- 20. Oxidation of alcohols
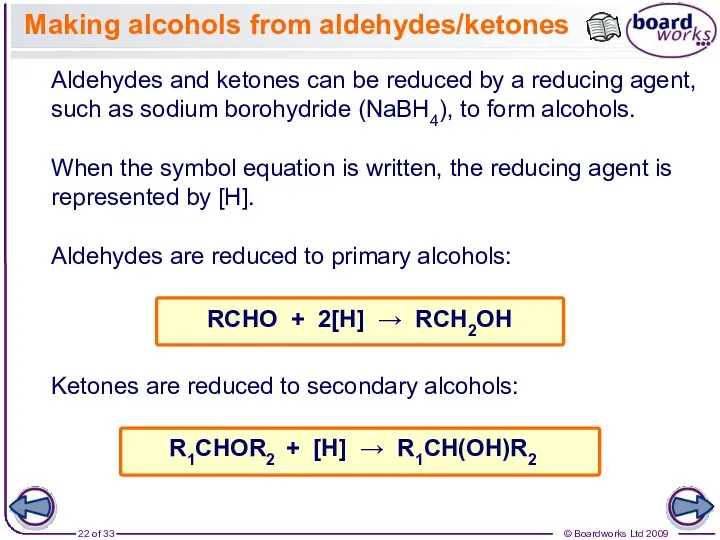
- 22. Making alcohols from aldehydes/ketones Aldehydes and ketones can be reduced by a reducing agent, such as
- 23. Synthesis of ethene from ethanol
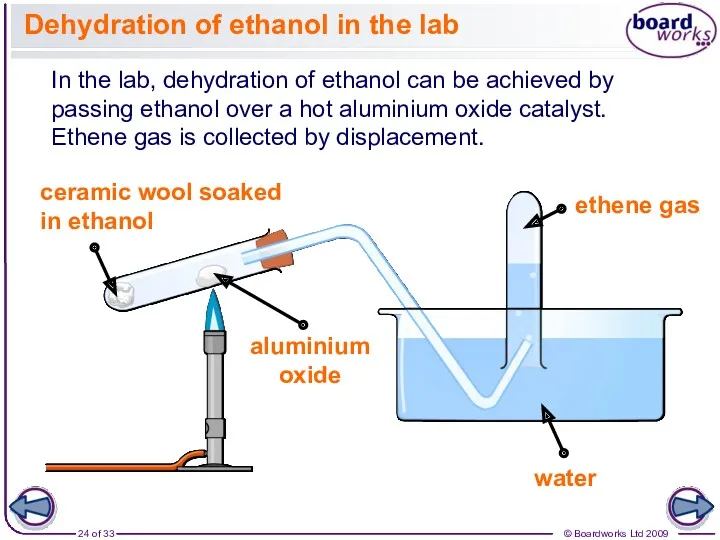
- 24. Dehydration of ethanol in the lab In the lab, dehydration of ethanol can be achieved by
- 25. Combustion of alcohols Alcohols undergo complete combustion to form carbon dioxide and water. Denatured alcohol is
- 26. Reaction with sodium
- 27. Forming halogenoalkanes from alcohols Primary, secondary and tertiary alcohols all react with phosphorus(V) chloride to form
- 28. Alcohol reactions
- 30. Glossary
- 31. What’s the keyword?
- 32. What’s the structure?
- 33. Multiple-choice quiz
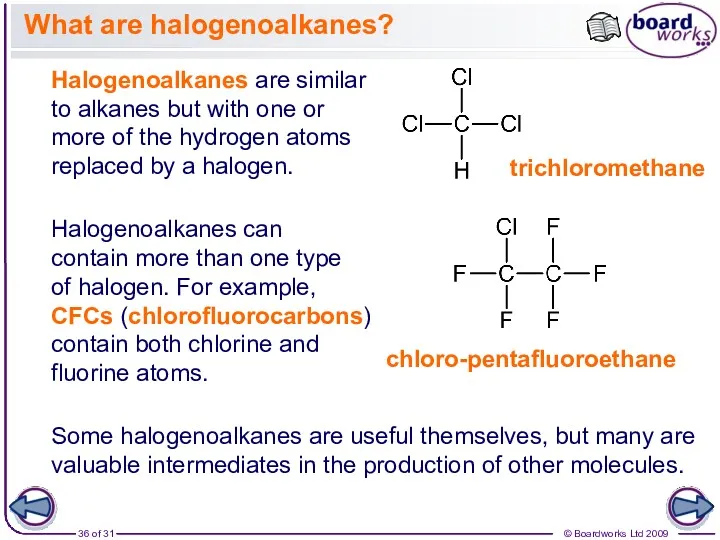
- 36. What are halogenoalkanes? Halogenoalkanes are similar to alkanes but with one or more of the hydrogen
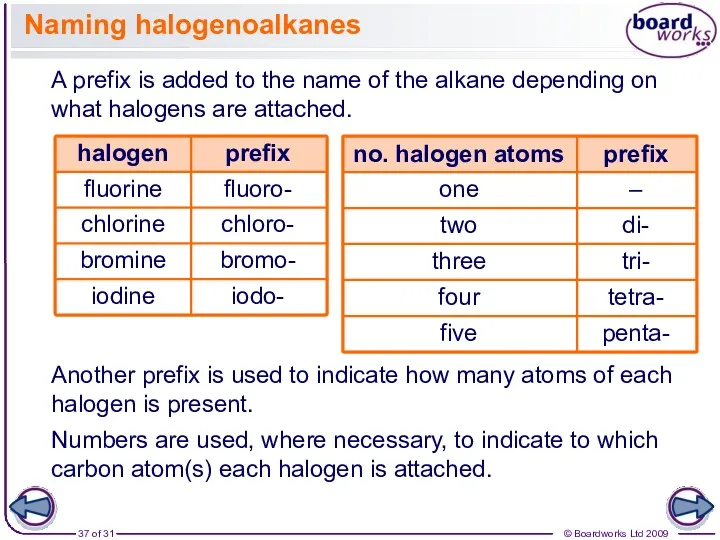
- 37. Naming halogenoalkanes A prefix is added to the name of the alkane depending on what halogens
- 38. What’s the halogenoalkane?
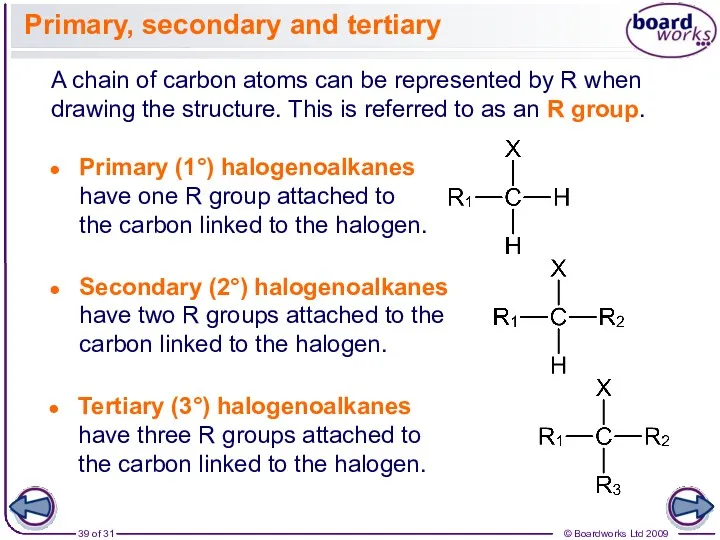
- 39. A chain of carbon atoms can be represented by R when drawing the structure. This is
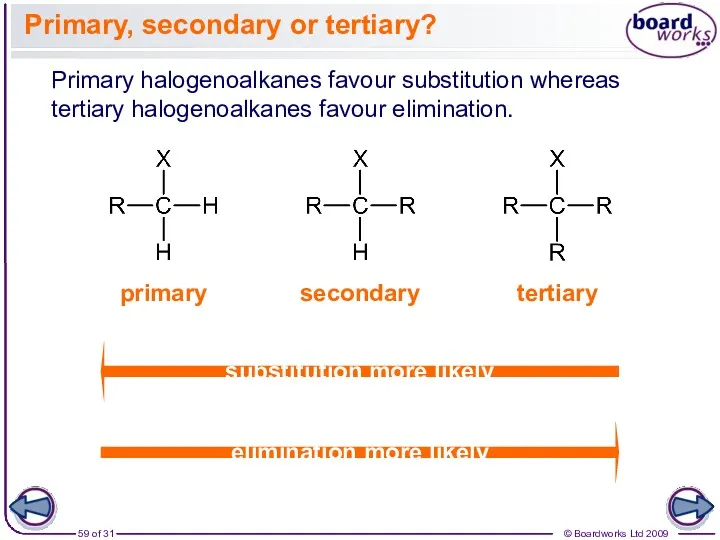
- 40. Primary, secondary or tertiary?
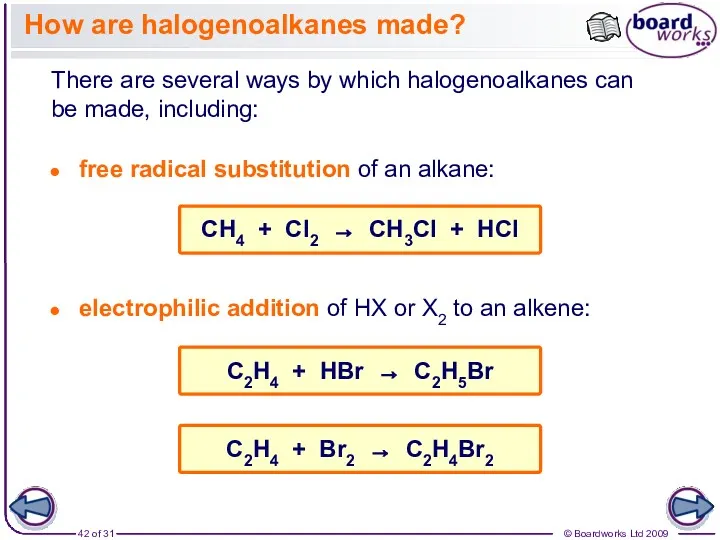
- 42. How are halogenoalkanes made? There are several ways by which halogenoalkanes can be made, including: free
- 43. Free radical substitution: Cl2 + CH4
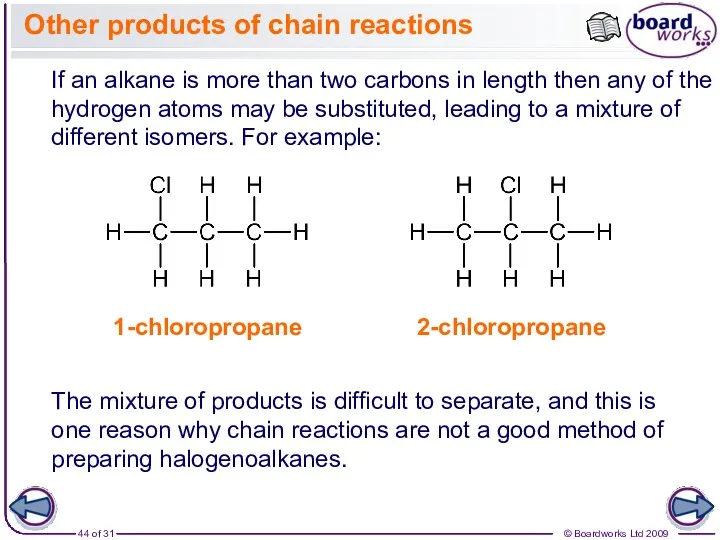
- 44. Other products of chain reactions If an alkane is more than two carbons in length then
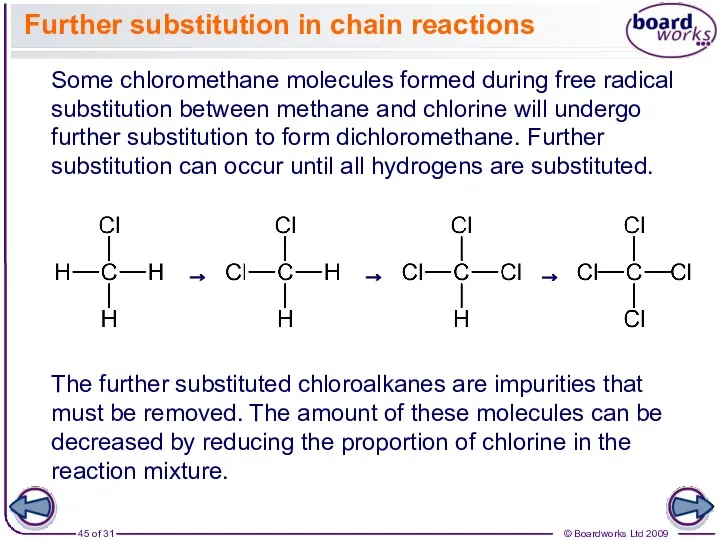
- 45. Further substitution in chain reactions Some chloromethane molecules formed during free radical substitution between methane and
- 46. Chain reactions and ozone
- 47. Free radical reactions: true or false?
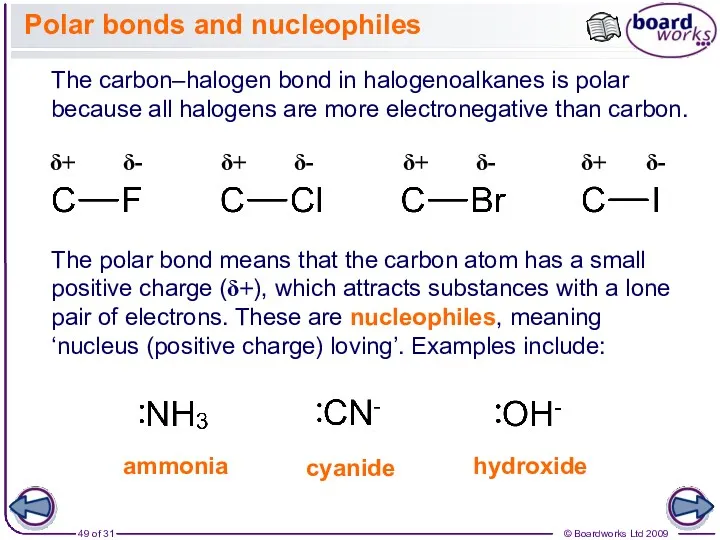
- 49. Polar bonds and nucleophiles The carbon–halogen bond in halogenoalkanes is polar because all halogens are more
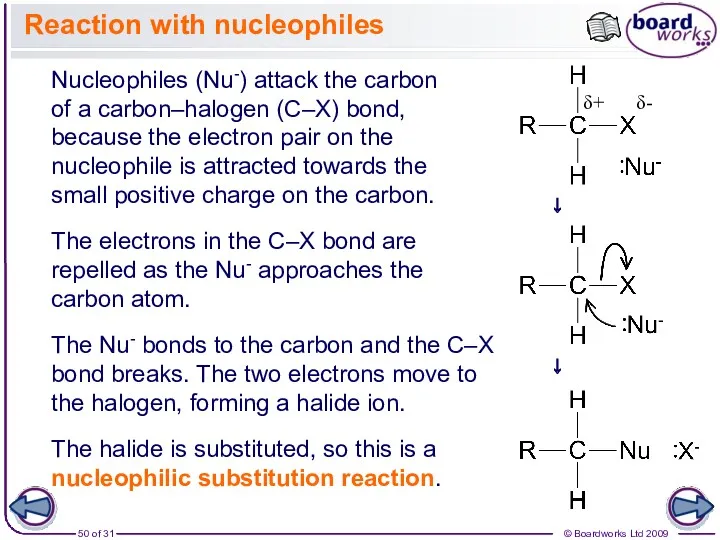
- 50. Nucleophiles (Nu-) attack the carbon of a carbon–halogen (C–X) bond, because the electron pair on the
- 51. Nucleophilic substitution reactions
- 52. Rate of nucleophilic substitution The rate of a nucleophilic substitution reaction depends on the strength of
- 53. Nucleophilic substitution
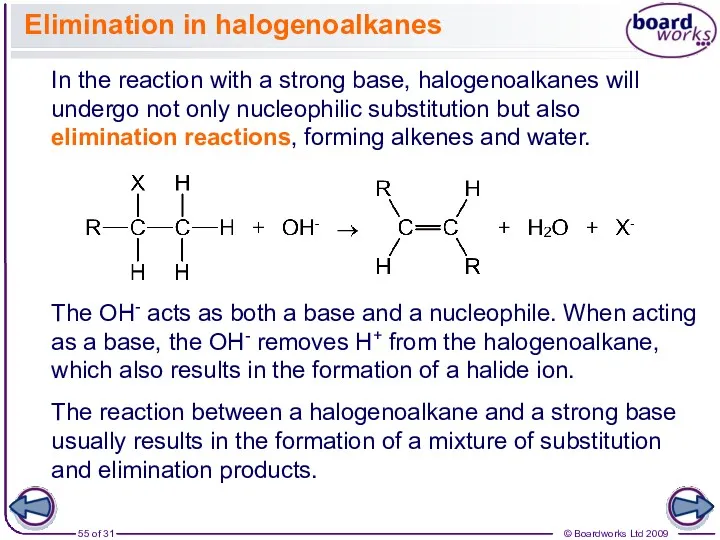
- 55. Elimination in halogenoalkanes In the reaction with a strong base, halogenoalkanes will undergo not only nucleophilic
- 56. Elimination mechanism
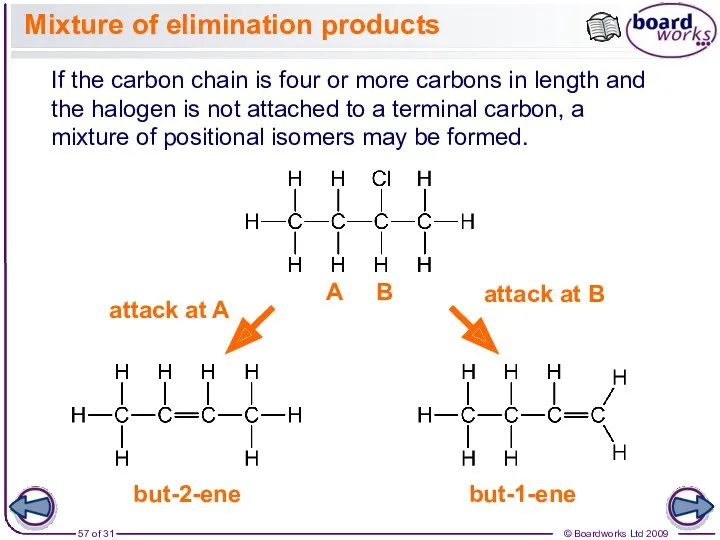
- 57. Mixture of elimination products If the carbon chain is four or more carbons in length and
- 58. Conditions are important The conditions for the reaction that favour substitution or elimination are different. Base
- 59. Primary, secondary or tertiary? Primary halogenoalkanes favour substitution whereas tertiary halogenoalkanes favour elimination. primary tertiary secondary
- 60. Elimination or substitution?
- 62. Glossary
- 63. What’s the keyword?
- 65. Скачать презентацию




































 Алкадиены (диеновые углеводороды)
Алкадиены (диеновые углеводороды) Атом - сложная частица
Атом - сложная частица Расчеты по химическим уравнениям. 8 класс
Расчеты по химическим уравнениям. 8 класс Темір және оның маңызды қосылыстары
Темір және оның маңызды қосылыстары Степень окисления
Степень окисления Роль воды в химических реакциях
Роль воды в химических реакциях Biochemistry. What is biochemistry?
Biochemistry. What is biochemistry? Кристалы и их свойства
Кристалы и их свойства Пируватдегидрогеназный комплекс
Пируватдегидрогеназный комплекс Электроизоляционные пластмассы
Электроизоляционные пластмассы 20230419_eds
20230419_eds Кислоты. Определение и классификация
Кислоты. Определение и классификация Химическая связь. Лекция 2-3
Химическая связь. Лекция 2-3 Снег и лед. Тайны твердой воды
Снег и лед. Тайны твердой воды Теоретические аспекты химического осаждения из газовой фазы
Теоретические аспекты химического осаждения из газовой фазы Сағыз пайдалы ма, әлде зиян ба?
Сағыз пайдалы ма, әлде зиян ба? Азотные удобрения
Азотные удобрения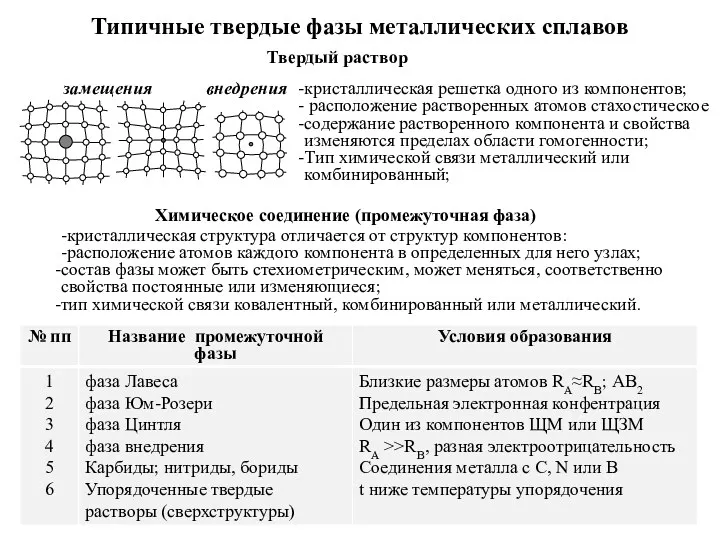 Типичные твердые фазы металлических сплавов
Типичные твердые фазы металлических сплавов Периодический закон и периодическая система химических элементов Д.И. Менделеева (8 класс)
Периодический закон и периодическая система химических элементов Д.И. Менделеева (8 класс) Генетическая связь
Генетическая связь Історія відкриття періодичної системи хімічних елементів
Історія відкриття періодичної системи хімічних елементів Периодический закон и ПСХЭ Д. И. Менделеева в свете учения о строении атома
Периодический закон и ПСХЭ Д. И. Менделеева в свете учения о строении атома Экзаменационные вопросы. Химическая связь в твердых телах
Экзаменационные вопросы. Химическая связь в твердых телах Карбонатна кислота. Солі карбонатної кислоти, їх поширення та застосування
Карбонатна кислота. Солі карбонатної кислоти, їх поширення та застосування Чистые вещества и смеси (продолжение)
Чистые вещества и смеси (продолжение) Основные классы неорганических веществ
Основные классы неорганических веществ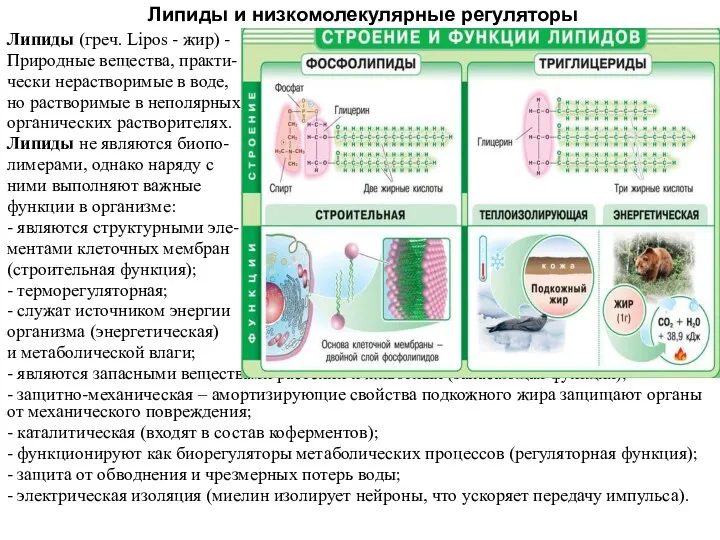 Липиды и низкомолекулярные регуляторы
Липиды и низкомолекулярные регуляторы Синтетичні високомолекулярні речовини. Полімери. Реакції полімеризації і поліконденсації
Синтетичні високомолекулярні речовини. Полімери. Реакції полімеризації і поліконденсації