Содержание
- 2. Chapter 7 Section1: Structure of the Atom
- 3. You will learn how to….. Compute the atomic mass and mass number of an atom Identify
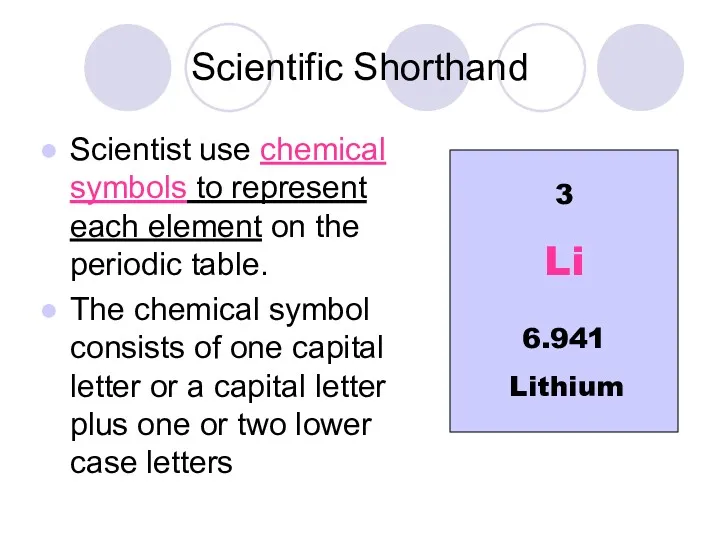
- 4. Scientific Shorthand Scientist use chemical symbols to represent each element on the periodic table. The chemical
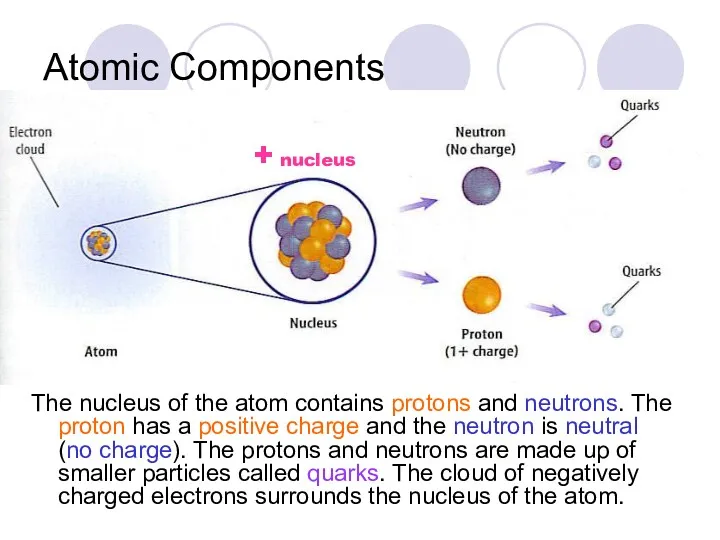
- 5. Atomic Components The nucleus of the atom contains protons and neutrons. The proton has a positive
- 6. The nucleus of the atom contains protons and neutrons. The proton has a positive charge the
- 7. The changing atomic model Scientists use models to represent things that are difficult to visualize ---or
- 8. The changing atomic model RECALL…..Matter is anything that has mass and takes up space…. EVERYTHING is
- 9. The changing atomic model John Dalton (1800s) Dalton’s Atomic Theory: All matter is made up of
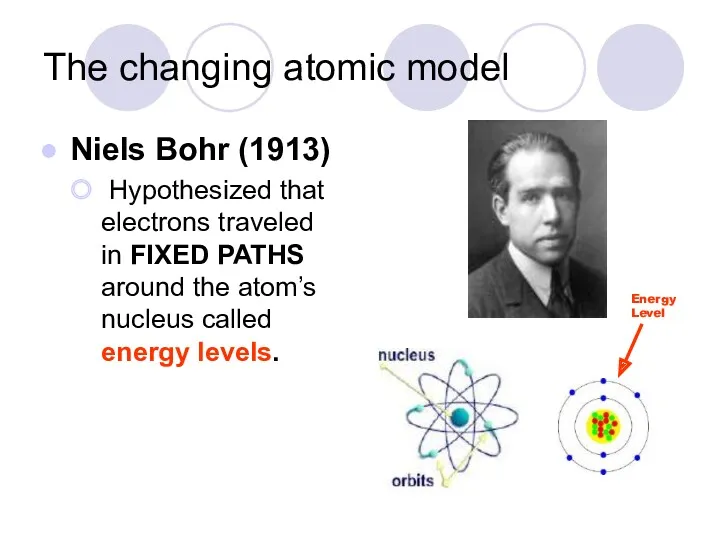
- 10. The changing atomic model Niels Bohr (1913) Hypothesized that electrons traveled in FIXED PATHS around the
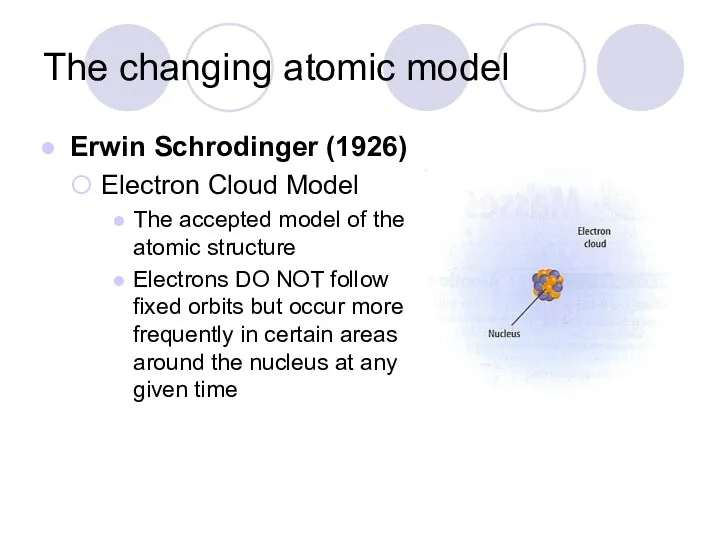
- 11. The changing atomic model Erwin Schrodinger (1926) Electron Cloud Model The accepted model of the atomic
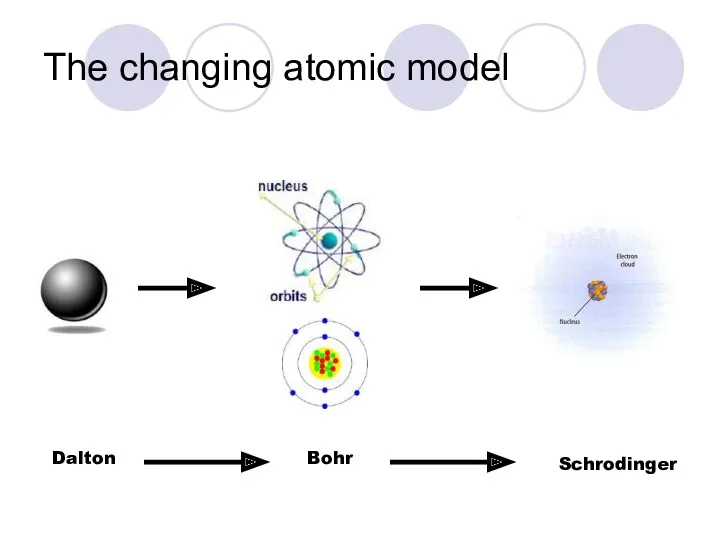
- 12. The changing atomic model Dalton Bohr Schrodinger
- 13. Chapter 18 Section 2: Masses of Atoms
- 14. You will learn how to…….. Compute the atomic mass and mass number of an atom. Identify
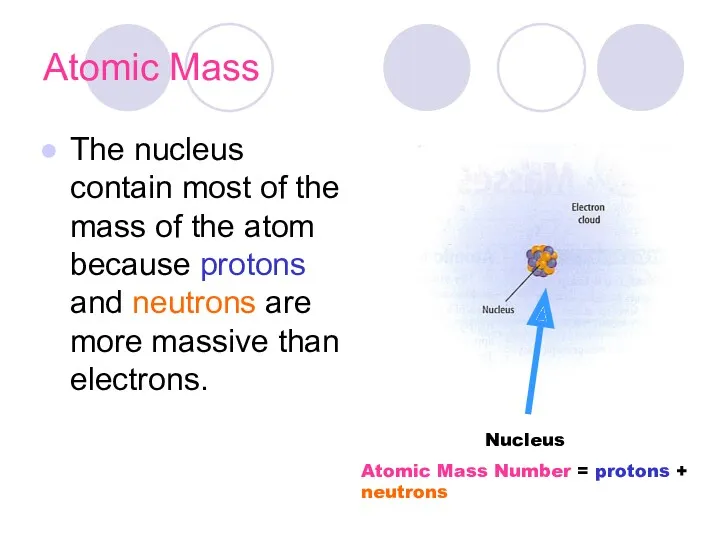
- 15. Atomic Mass The nucleus contain most of the mass of the atom because protons and neutrons
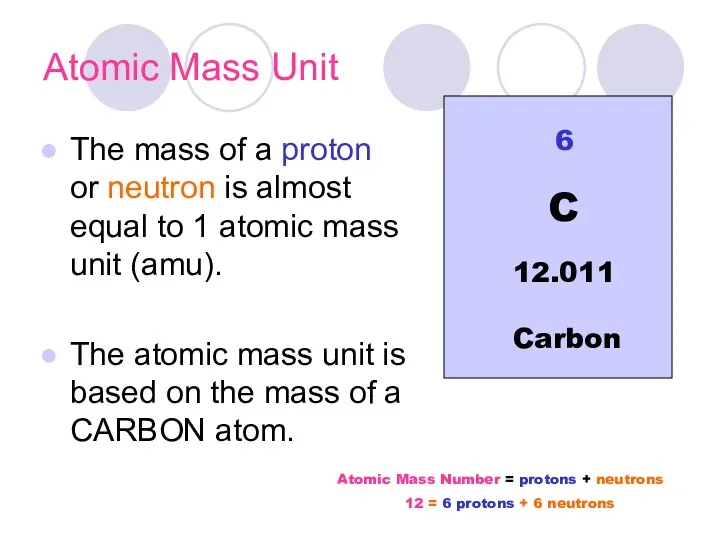
- 16. Atomic Mass Unit The mass of a proton or neutron is almost equal to 1 atomic
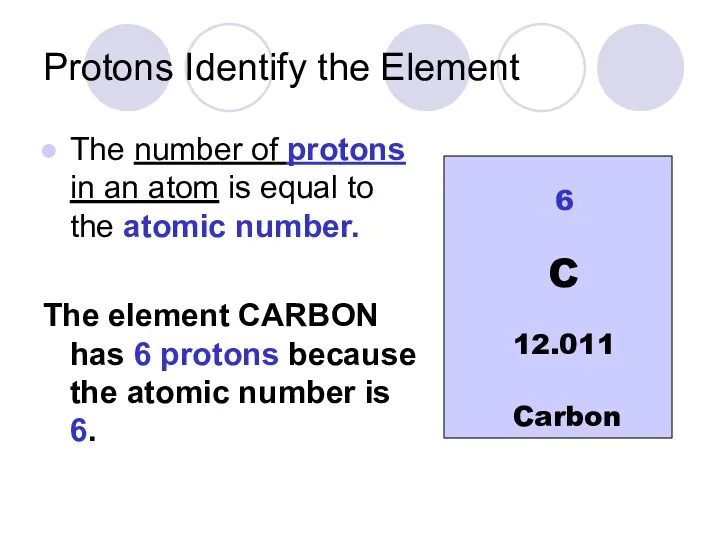
- 17. Protons Identify the Element The number of protons in an atom is equal to the atomic
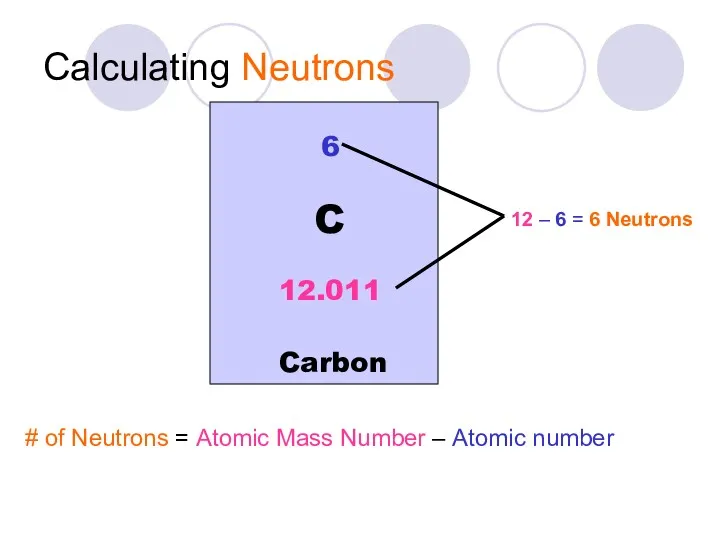
- 18. Calculating Neutrons # of Neutrons = Atomic Mass Number – Atomic number 6 Carbon C 12.011
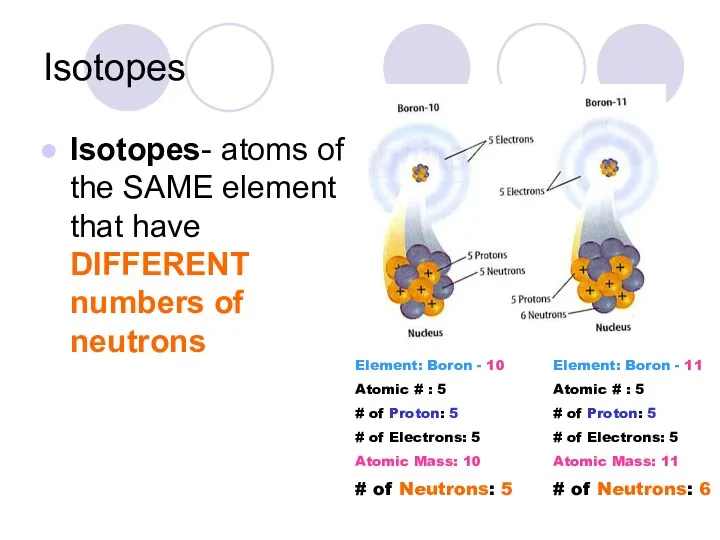
- 19. Isotopes Isotopes- atoms of the SAME element that have DIFFERENT numbers of neutrons Element: Boron -
- 20. Chapter 18 Section 3: The Periodic Table
- 21. You will learn how to…… Explain the composition of the periodic table. Use the periodic table
- 22. The Periodic Table Periodic means “repeated in a pattern” Ex. The calendar: the days repeat every
- 23. Dmitri Mendeleev (1834-1907) constructed the FIRST periodic table he listed the elements in columns in order
- 24. Henry Moseley (1913) a British physicist who determined the atomic number of the atoms of the
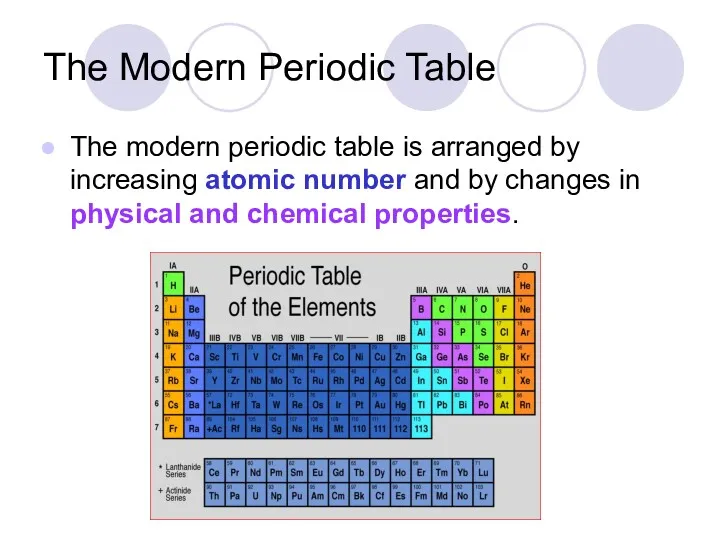
- 25. The Modern Periodic Table The modern periodic table is arranged by increasing atomic number and by
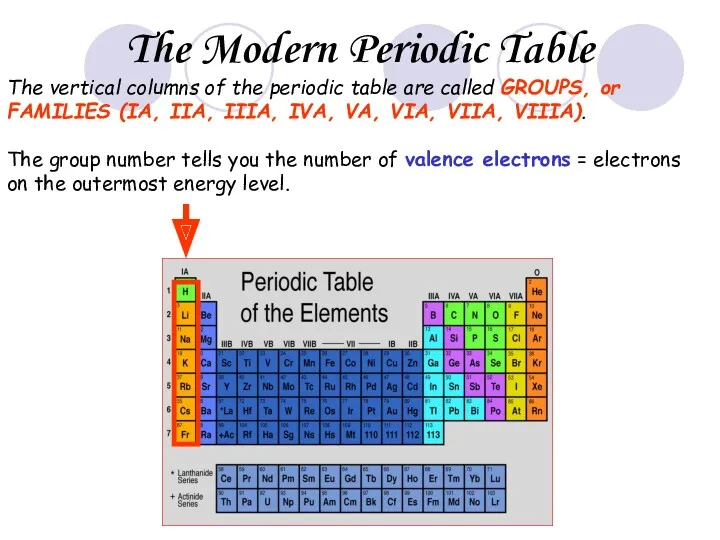
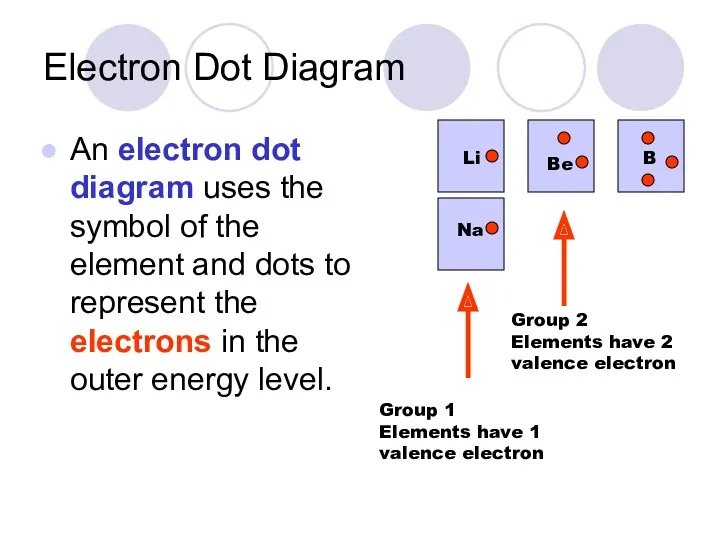
- 26. The vertical columns of the periodic table are called GROUPS, or FAMILIES (IA, IIA, IIIA, IVA,
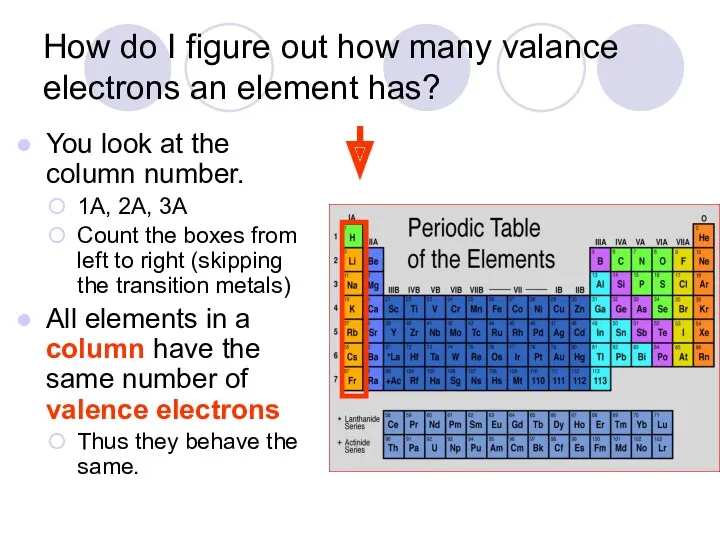
- 27. How do I figure out how many valance electrons an element has? You look at the
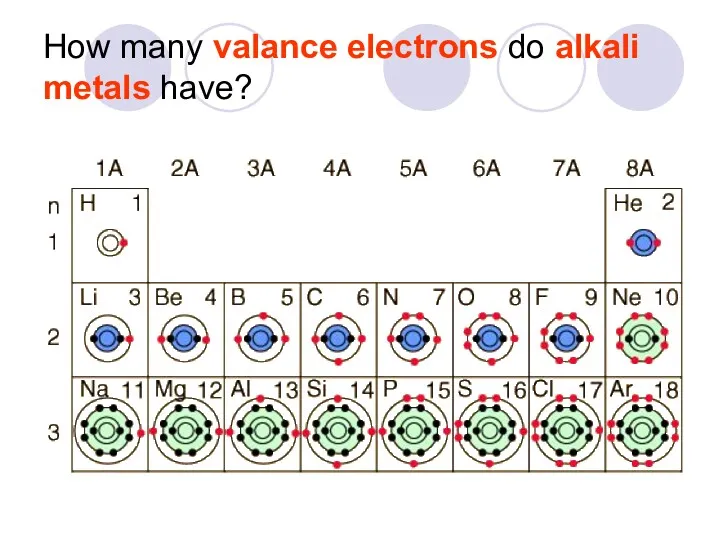
- 28. How many valance electrons do alkali metals have?
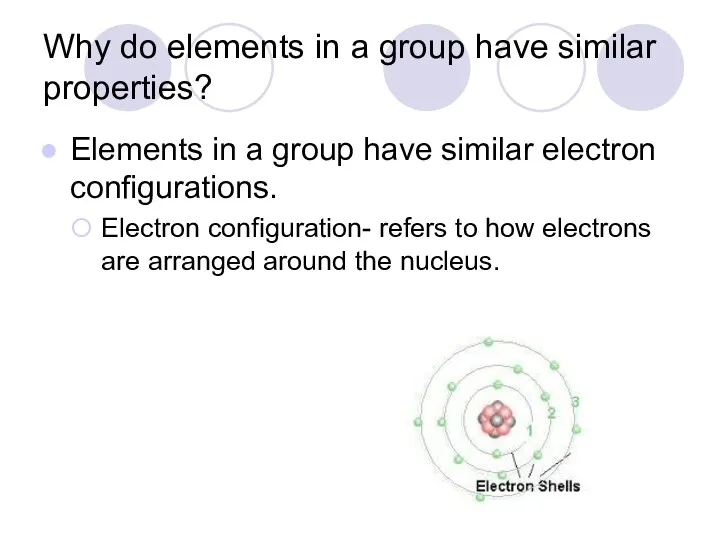
- 29. Why do elements in a group have similar properties? Elements in a group have similar electron
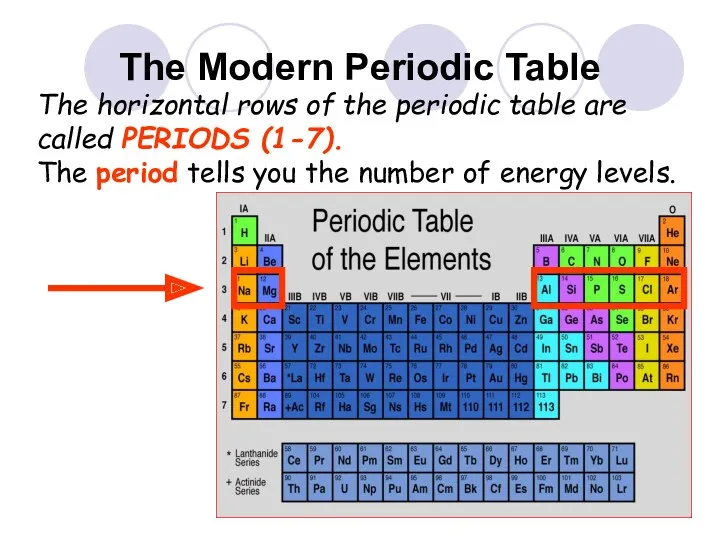
- 30. The Modern Periodic Table The horizontal rows of the periodic table are called PERIODS (1-7). The
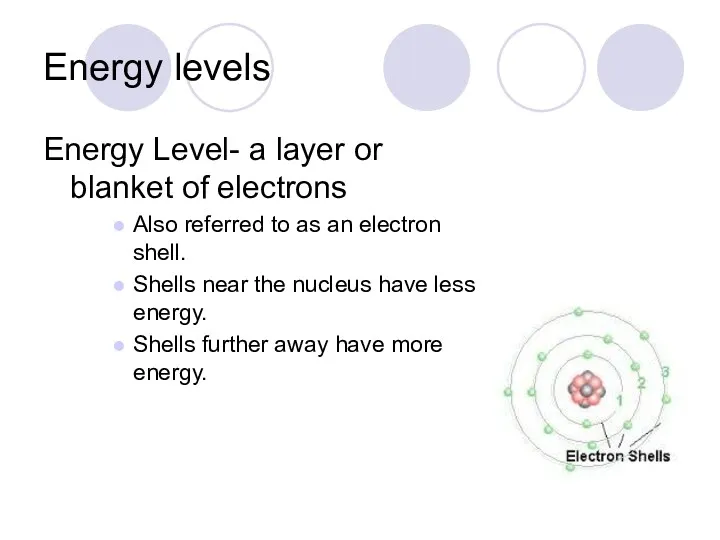
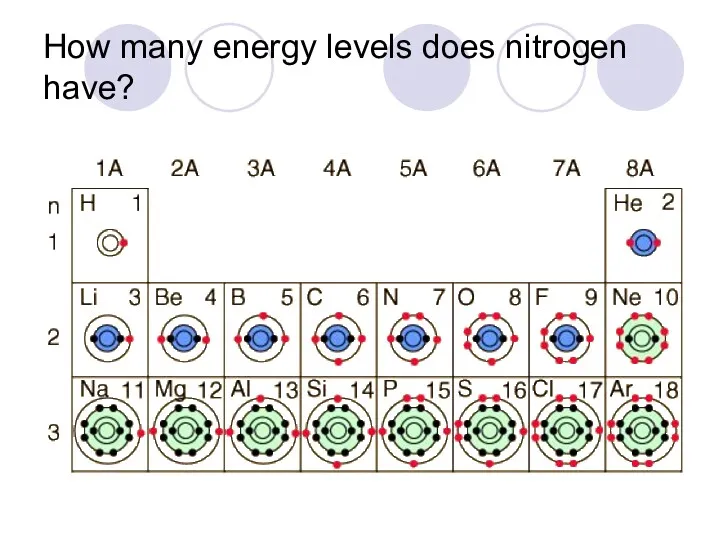
- 31. Energy levels Energy Level- a layer or blanket of electrons Also referred to as an electron
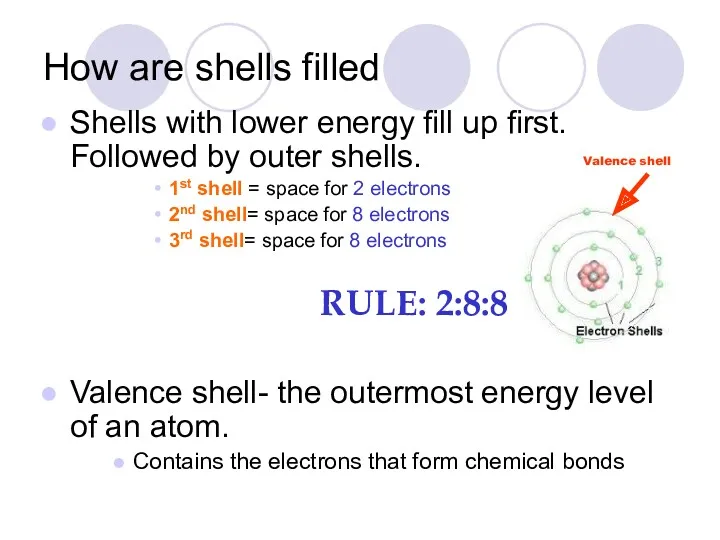
- 32. How are shells filled Shells with lower energy fill up first. Followed by outer shells. 1st
- 33. How do I figure out the number of shells on an atom? Each period adds another
- 34. How many energy levels does nitrogen have?
- 35. Electron Dot Diagram An electron dot diagram uses the symbol of the element and dots to
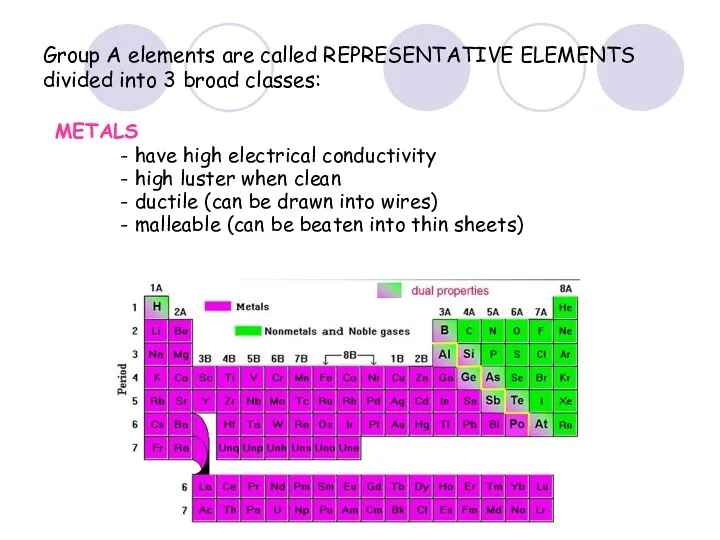
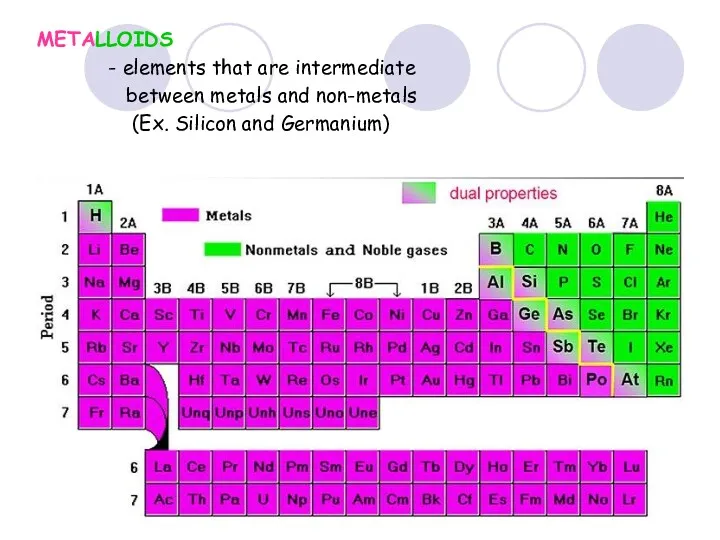
- 36. Group A elements are called REPRESENTATIVE ELEMENTS divided into 3 broad classes: METALS - have high
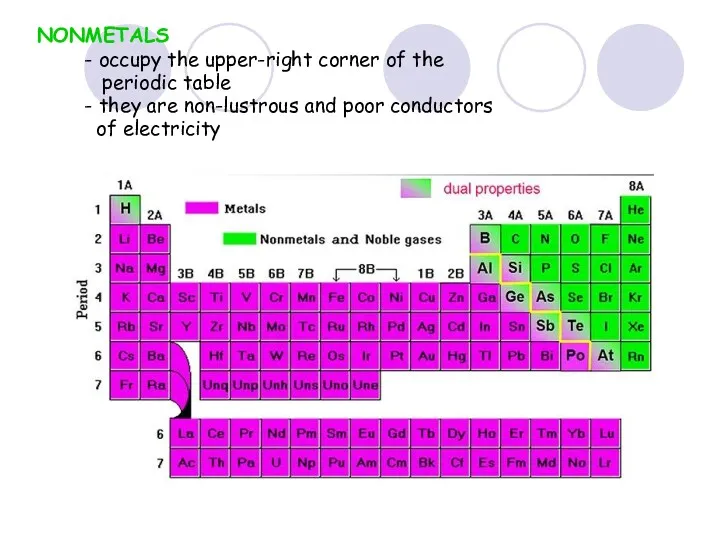
- 37. NONMETALS - occupy the upper-right corner of the periodic table - they are non-lustrous and poor
- 38. METALLOIDS - elements that are intermediate between metals and non-metals (Ex. Silicon and Germanium)
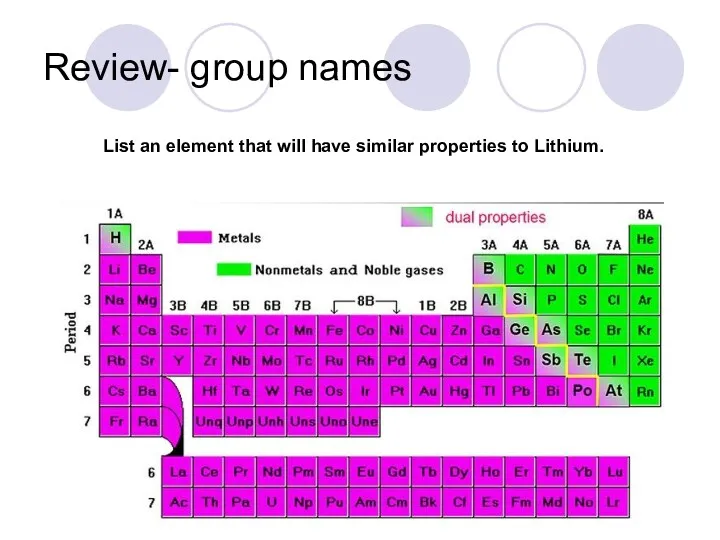
- 39. Review- group names List an element that will have similar properties to Lithium.
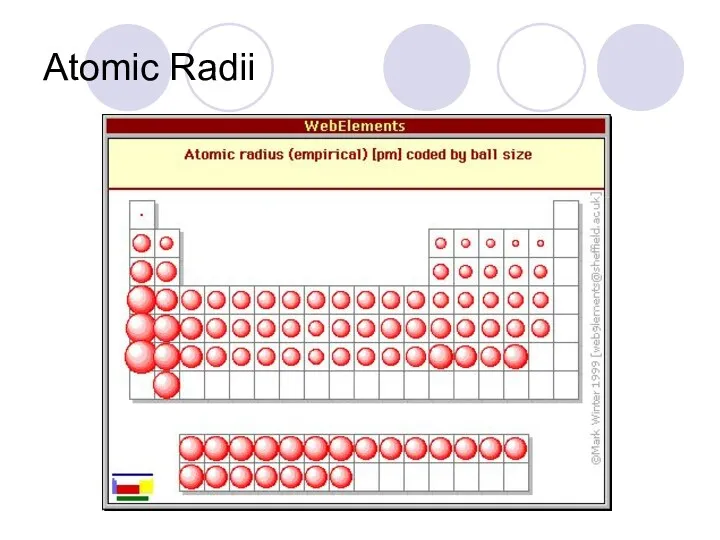
- 40. Periodic Trends Atomic radii- the size of an atom From top to bottom atoms get bigger
- 41. Atomic Radii
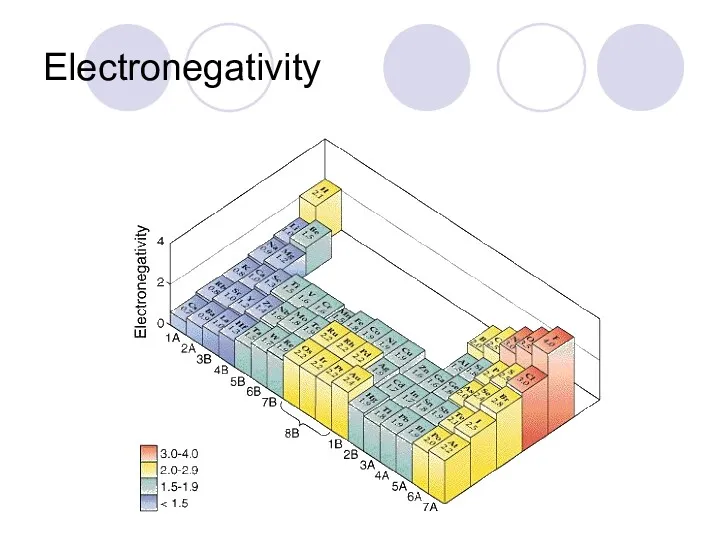
- 42. Electronegativity Electronegativity- ability to take electrons from another atom. From top to bottom- gets weaker From
- 43. Electronegativity
- 45. Скачать презентацию














 D-элементы: хром, молибден, вольфрам
D-элементы: хром, молибден, вольфрам Теория растворов. Диффузия в растворах. Коллигативные свойства растворов
Теория растворов. Диффузия в растворах. Коллигативные свойства растворов Игра по химии В рамках периодической таблицы
Игра по химии В рамках периодической таблицы Классификация химических реакций
Классификация химических реакций Непредельные углеводороды. Алкины
Непредельные углеводороды. Алкины Нуклеозиды. Нуклеиновые кислоты
Нуклеозиды. Нуклеиновые кислоты Полимеры
Полимеры Нефть. Способы её переработки. Фракции нефти
Нефть. Способы её переработки. Фракции нефти Жёсткость воды и способы её устранения
Жёсткость воды и способы её устранения Применение алканов
Применение алканов Основы химического равновесия
Основы химического равновесия Горючее, смазочные материалы и специальные жидкости
Горючее, смазочные материалы и специальные жидкости Кислородсодержащие соединения. Ароматические соединения
Кислородсодержащие соединения. Ароматические соединения Классификация полимеров
Классификация полимеров Спектроскопія ямр на ядрах 13с
Спектроскопія ямр на ядрах 13с Альдегіди. Карбонові кислоти. Одержання. Фізичні та хімічні властивості (10 клас)
Альдегіди. Карбонові кислоти. Одержання. Фізичні та хімічні властивості (10 клас) Скорость химических реакций. Факторы, влияющие на скорость химической реакции
Скорость химических реакций. Факторы, влияющие на скорость химической реакции Свойства жидких металлов
Свойства жидких металлов Основные классы неорганических соединений. Тема 2
Основные классы неорганических соединений. Тема 2 Алюминий. Строение
Алюминий. Строение Обмен нуклеотидов
Обмен нуклеотидов Классификация, строение и номенклатура органически. Предмет органической химии
Классификация, строение и номенклатура органически. Предмет органической химии Органические производные титана со связью Ti-C
Органические производные титана со связью Ti-C Получение каталитического слоя на основе углеродных нанотрубок с наночастицами платины для водородно–воздушных топливных элементов
Получение каталитического слоя на основе углеродных нанотрубок с наночастицами платины для водородно–воздушных топливных элементов Биосинтез и катаболизм пуриновых и пиримидиновых нуклеотидов
Биосинтез и катаболизм пуриновых и пиримидиновых нуклеотидов Непредельные углеводороды. Алкены
Непредельные углеводороды. Алкены Железо, его физические и химические свойства. Урок химии в 9 классе
Железо, его физические и химические свойства. Урок химии в 9 классе Фосфор и его соединения. Урок по химии для 9 класса
Фосфор и его соединения. Урок по химии для 9 класса