Содержание
- 2. LESSON OBJECTIVES: Concept of electrolytes Define electrolyte, electrolytic solution, ion, cation, anion Arrhenius theory of electrolytic
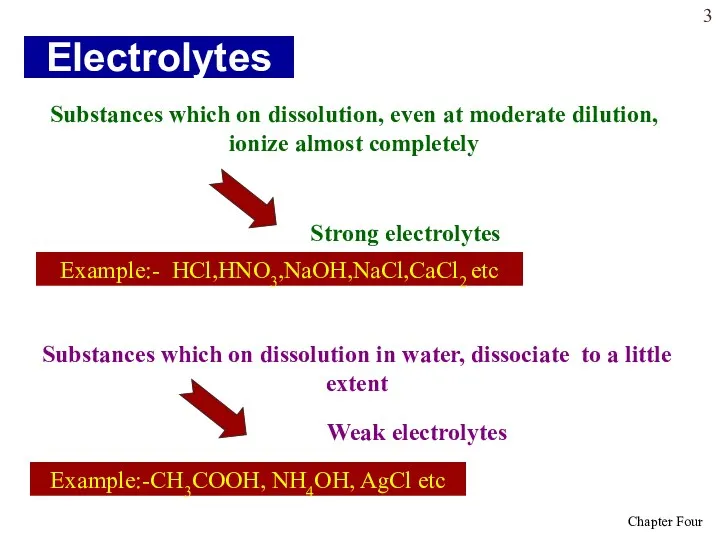
- 3. Electrolytes Substances which on dissolution, even at moderate dilution, ionize almost completely Substances which on dissolution
- 4. In the world of chemistry, an electrolyte is a substance having the free ions so that
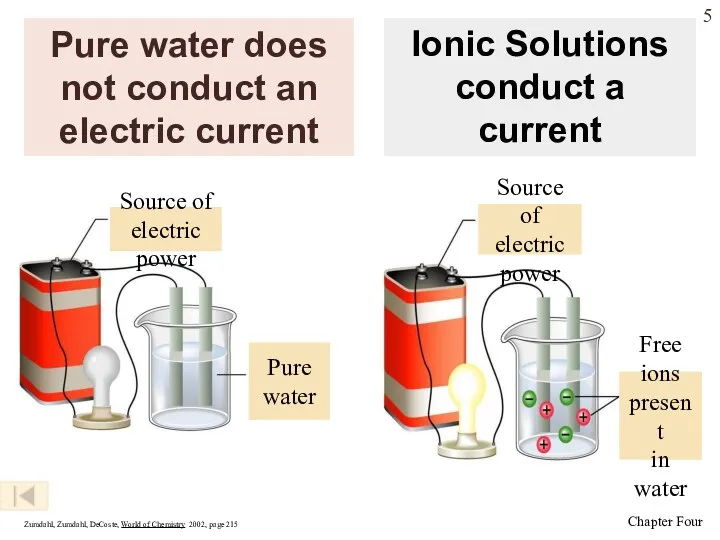
- 5. Pure water does not conduct an electric current Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page
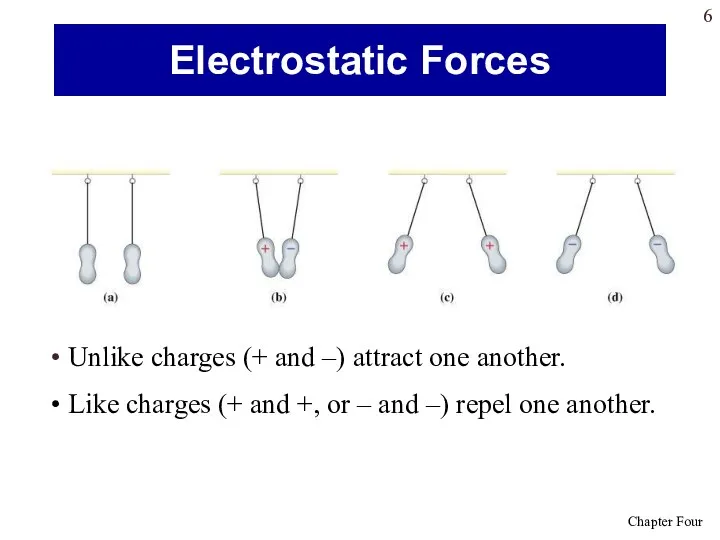
- 6. Unlike charges (+ and –) attract one another. Like charges (+ and +, or – and
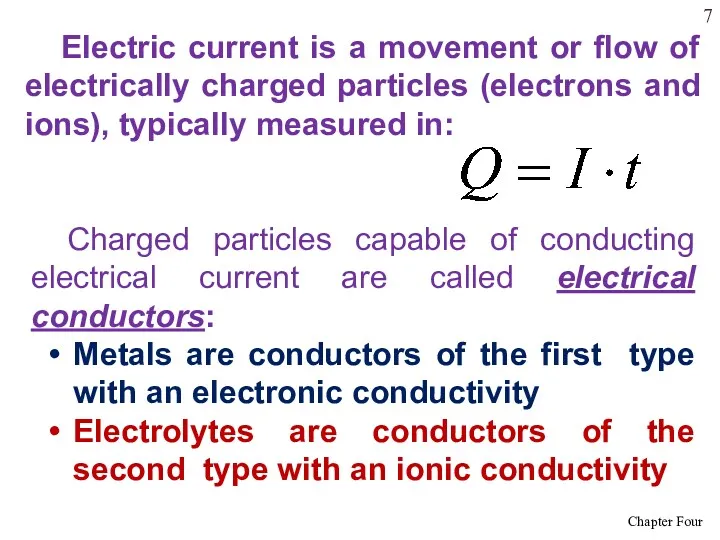
- 7. Electric current is a movement or flow of electrically charged particles (electrons and ions), typically measured
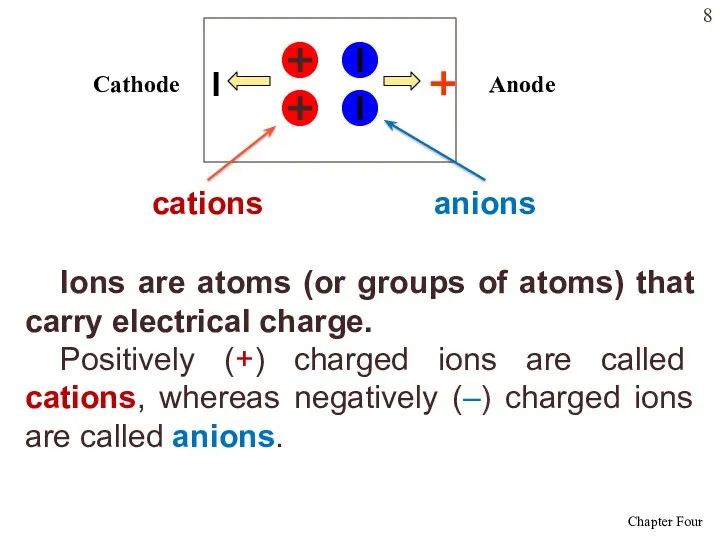
- 8. Ions are atoms (or groups of atoms) that carry electrical charge. Positively (+) charged ions are
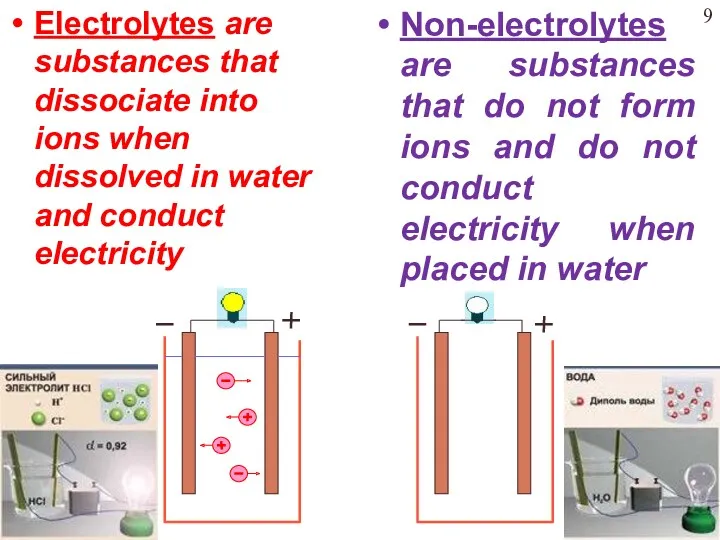
- 9. Electrolytes are substances that dissociate into ions when dissolved in water and conduct electricity Non-electrolytes are
- 10. Electrical Conductivity of Ionic Solutions
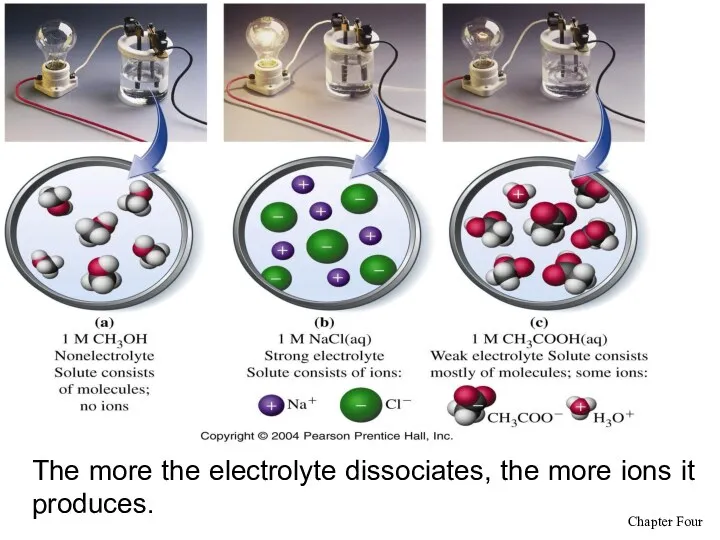
- 11. The more the electrolyte dissociates, the more ions it produces.
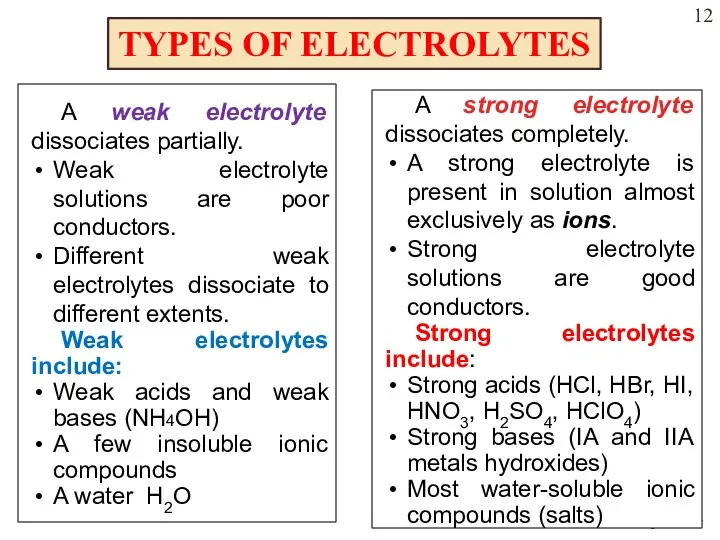
- 12. TYPES OF ELECTROLYTES A weak electrolyte dissociates partially. Weak electrolyte solutions are poor conductors. Different weak
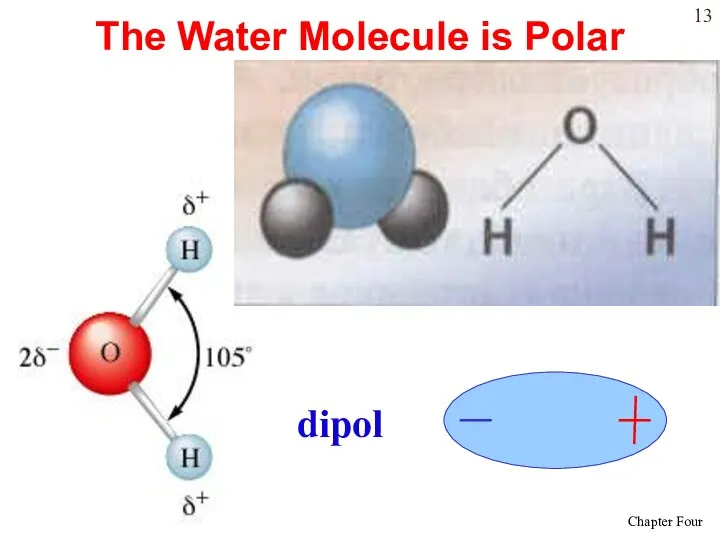
- 13. The Water Molecule is Polar dipol
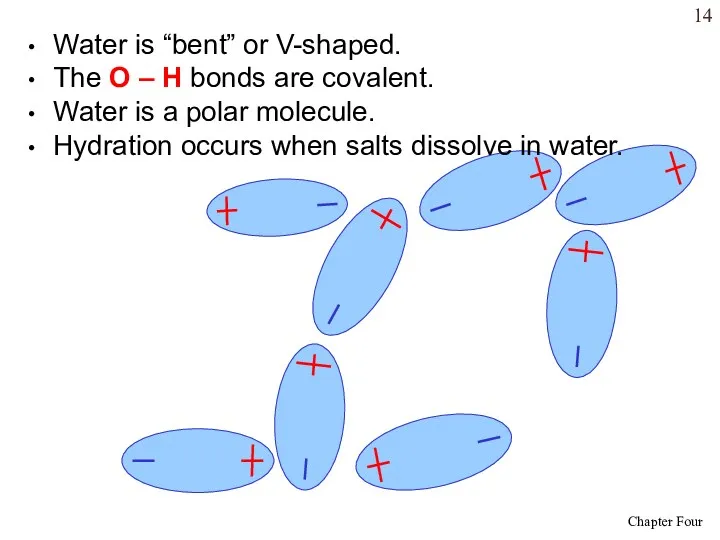
- 14. Water is “bent” or V-shaped. The O – H bonds are covalent. Water is a polar
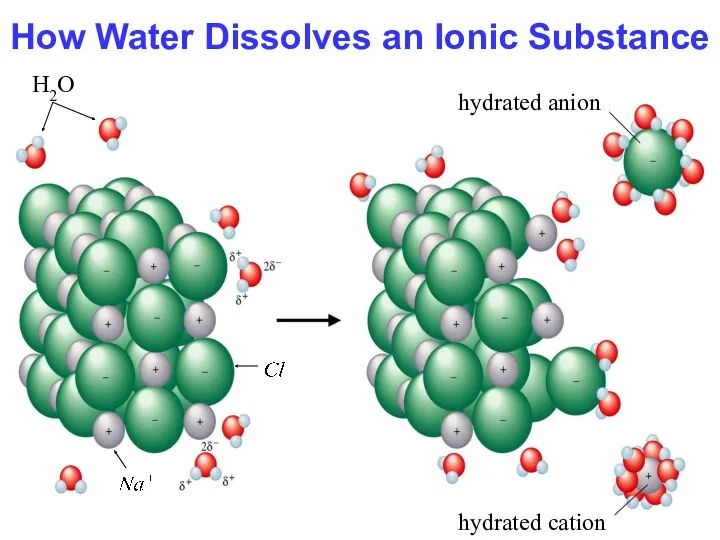
- 15. How Water Dissolves an Ionic Substance H2O hydrated cation hydrated anion
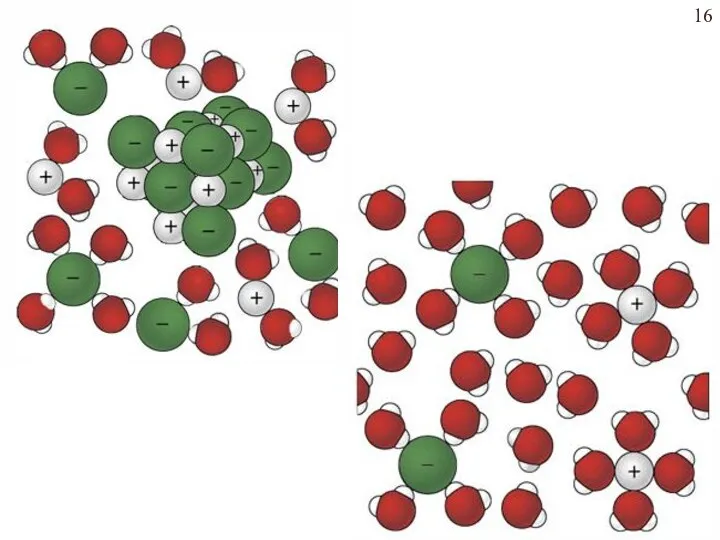
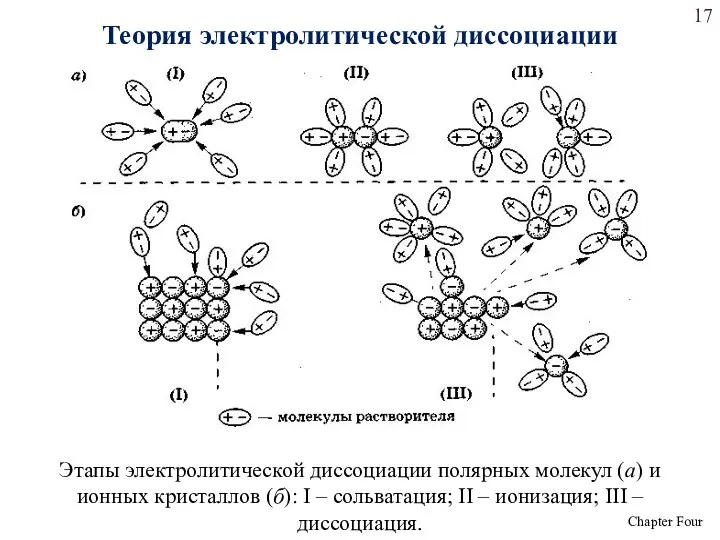
- 17. Этапы электролитической диссоциации полярных молекул (а) и ионных кристаллов (б): I – сольватация; II – ионизация;
- 18. In order to explain the properties of electrolytic solutions, Arrhenius put forth, in 1884, a comprehensive
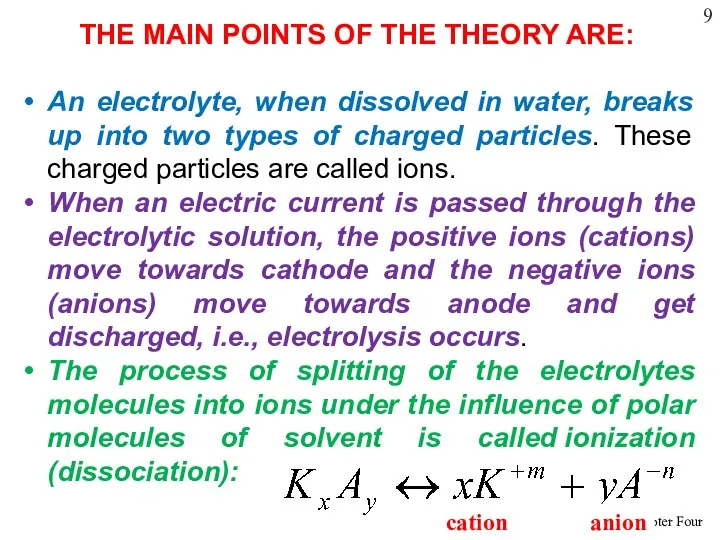
- 19. THE MAIN POINTS OF THE THEORY ARE: An electrolyte, when dissolved in water, breaks up into
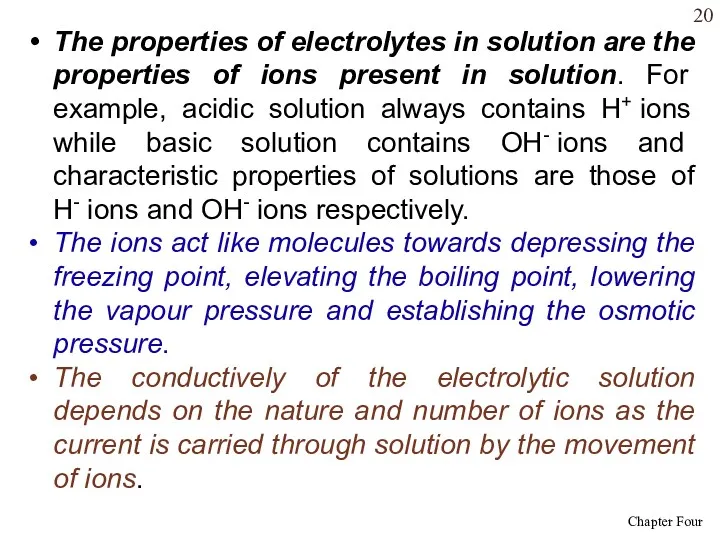
- 20. The properties of electrolytes in solution are the properties of ions present in solution. For example,
- 21. An acid is a substance that increase H+ when dissolved in water: Some acids have more
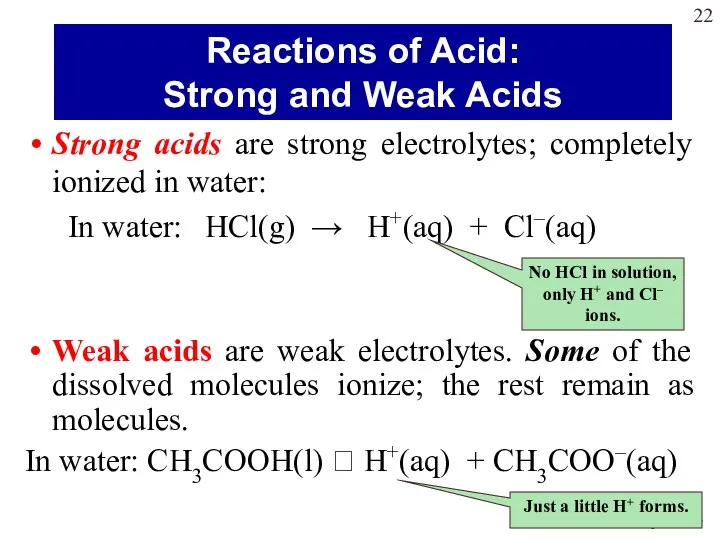
- 22. Strong acids are strong electrolytes; completely ionized in water: In water: HCl(g) → H+(aq) + Cl–(aq)
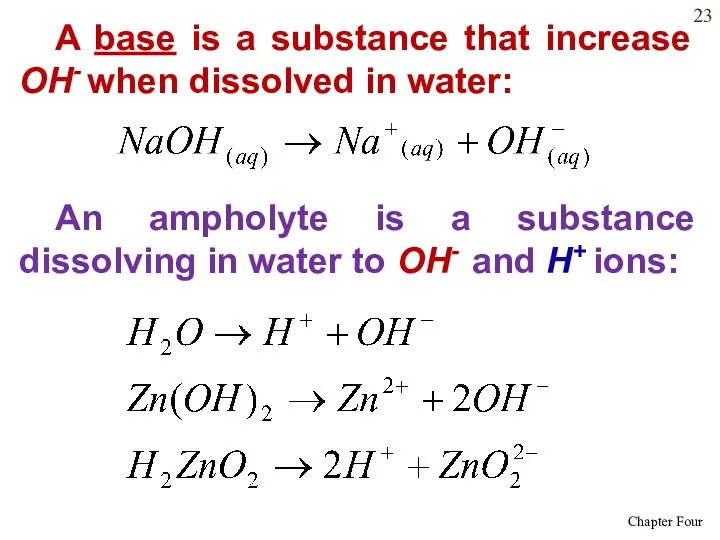
- 23. A base is a substance that increase OH- when dissolved in water: An ampholyte is a
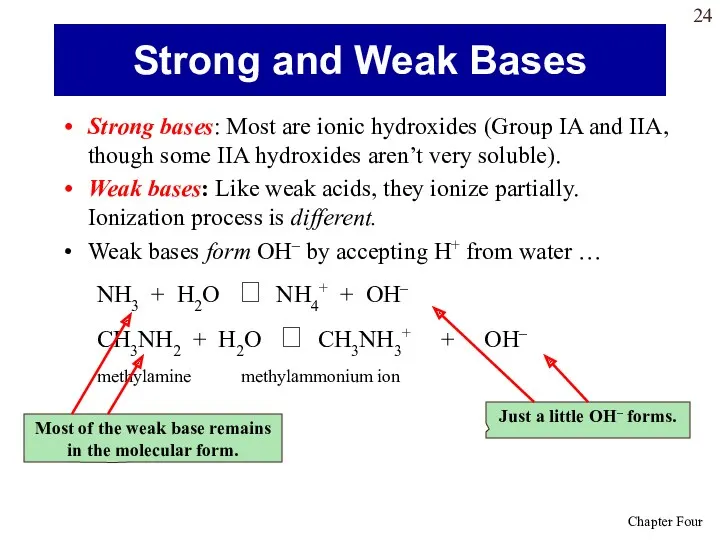
- 24. Strong bases: Most are ionic hydroxides (Group IA and IIA, though some IIA hydroxides aren’t very
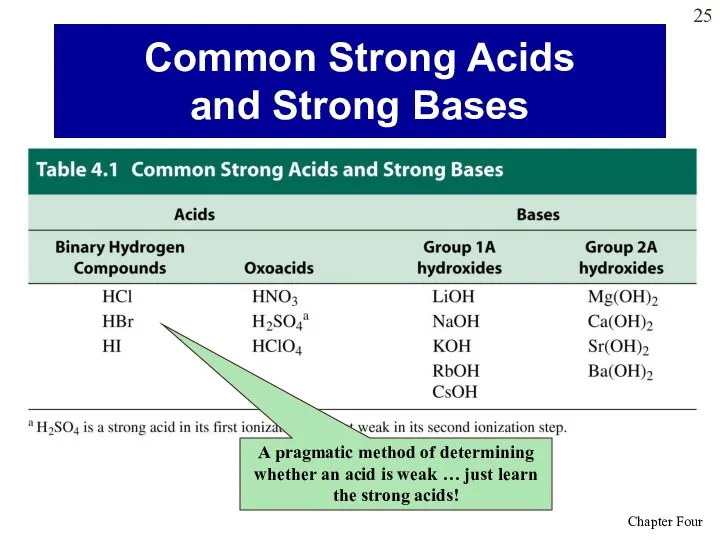
- 25. Common Strong Acids and Strong Bases A pragmatic method of determining whether an acid is weak
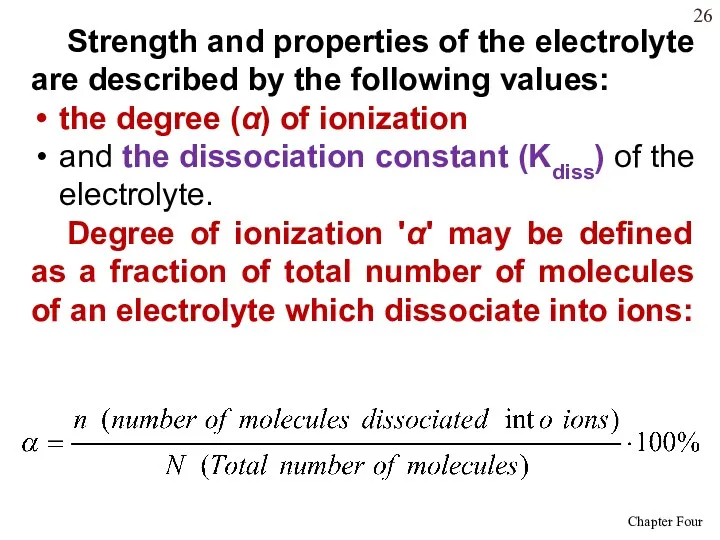
- 26. Strength and properties of the electrolyte are described by the following values: the degree (α) of
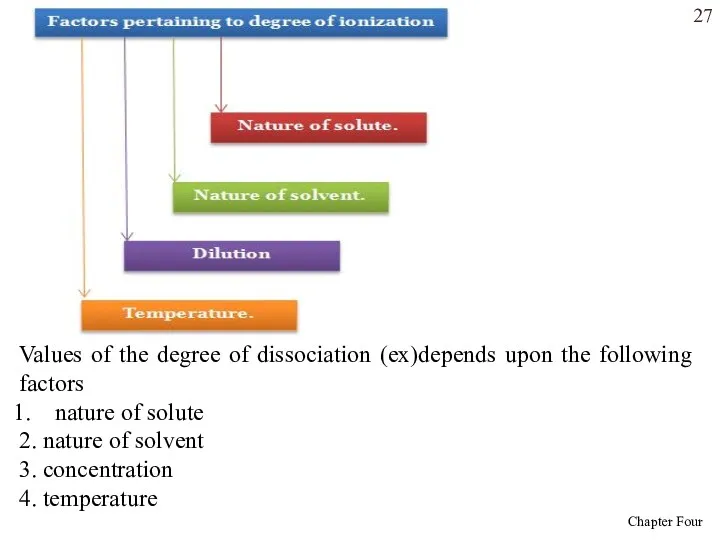
- 27. Values of the degree of dissociation (ex)depends upon the following factors nature of solute 2. nature
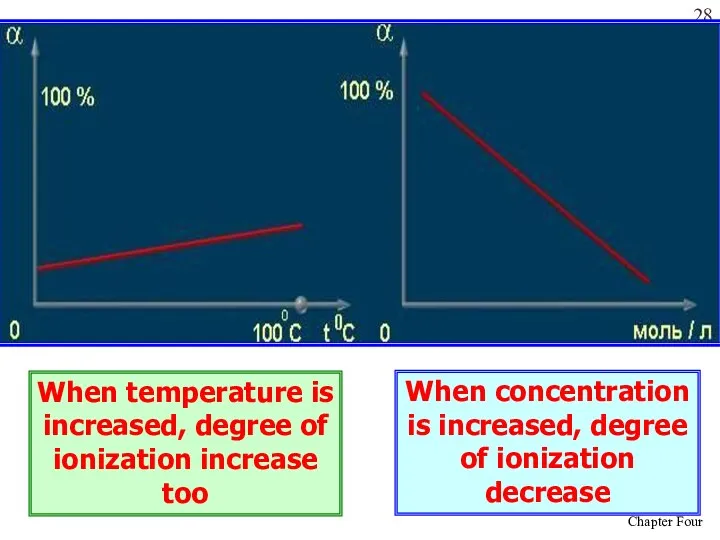
- 28. When temperature is increased, degree of ionization increase too When concentration is increased, degree of ionization
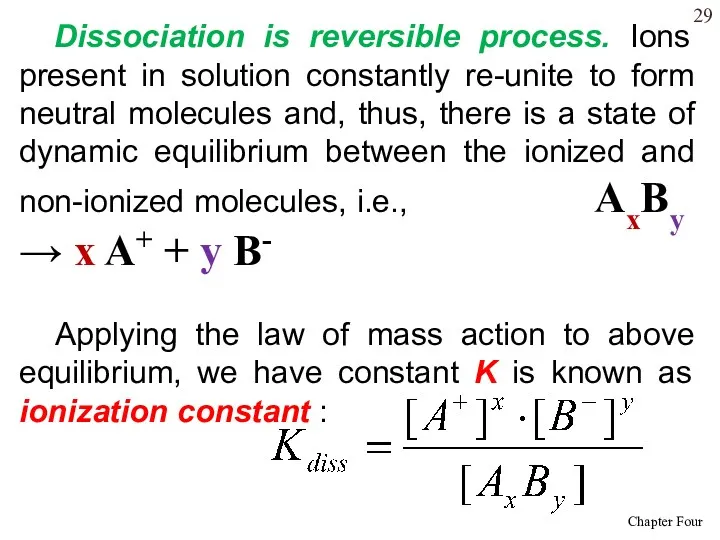
- 29. Dissociation is reversible process. Ions present in solution constantly re-unite to form neutral molecules and, thus,
- 30. For strong electrolytes α>0,3 (30%) and they having high value of Kdiss For weak electrolytes α
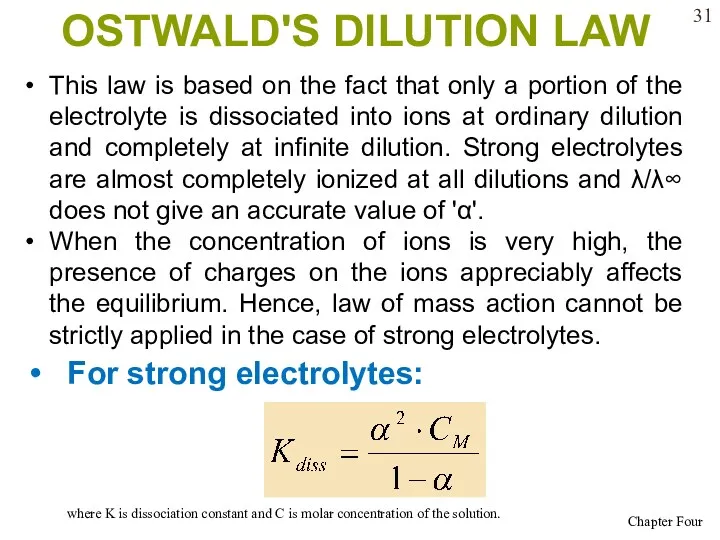
- 31. OSTWALD'S DILUTION LAW For strong electrolytes: where K is dissociation constant and C is molar concentration
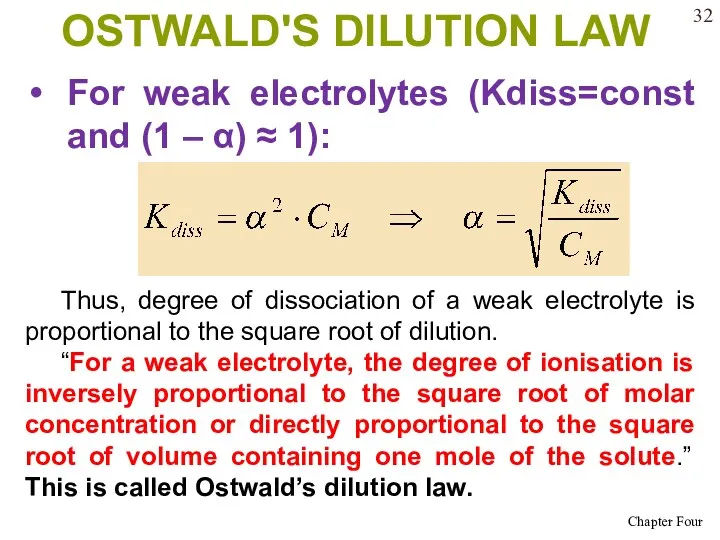
- 32. OSTWALD'S DILUTION LAW For weak electrolytes (Kdiss=const and (1 – α) ≈ 1): Thus, degree of
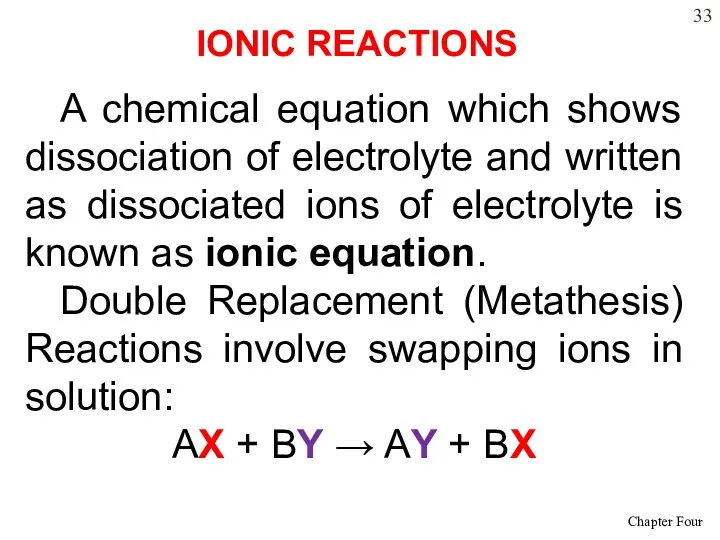
- 33. A chemical equation which shows dissociation of electrolyte and written as dissociated ions of electrolyte is
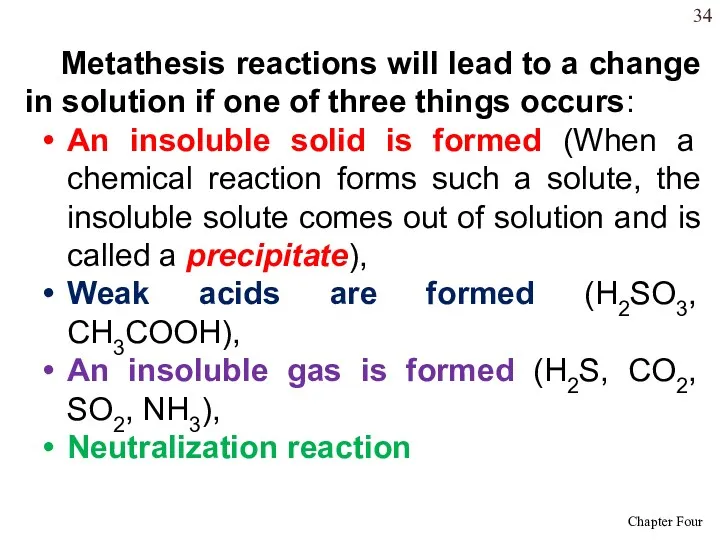
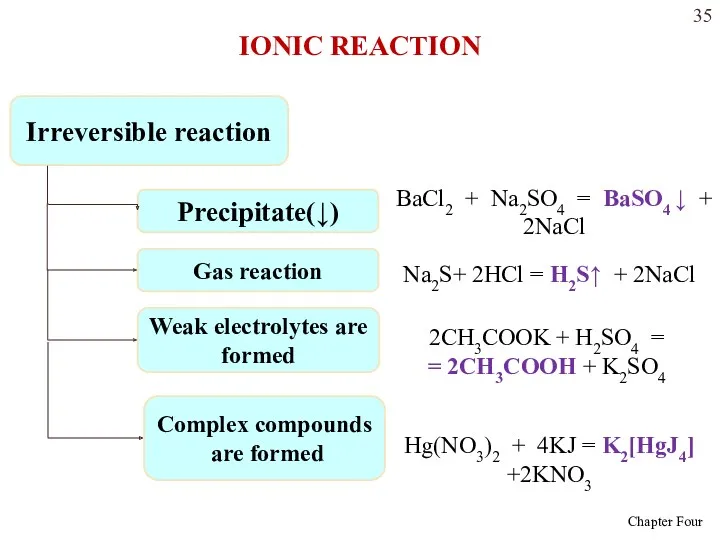
- 34. Metathesis reactions will lead to a change in solution if one of three things occurs: An
- 35. Irreversible reaction Precipitate(↓) BaCl2 + Na2SO4 = BaSO4 ↓ + 2NaCl Gas reaction Na2S+ 2HCl =
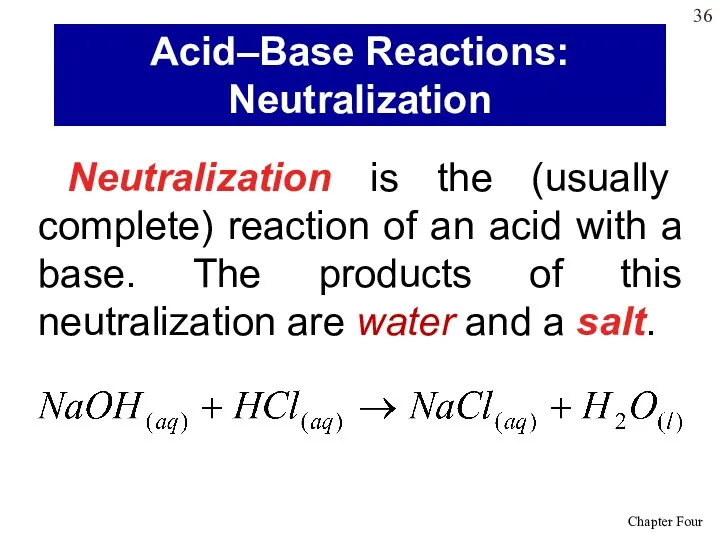
- 36. Neutralization is the (usually complete) reaction of an acid with a base. The products of this
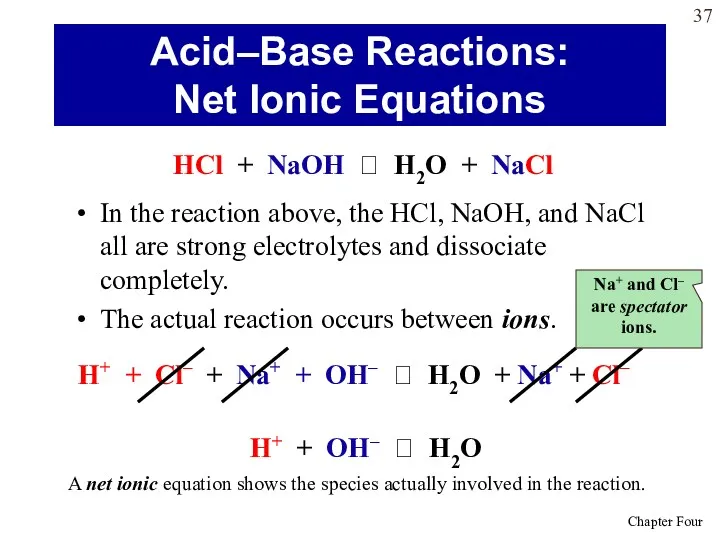
- 37. In the reaction above, the HCl, NaOH, and NaCl all are strong electrolytes and dissociate completely.
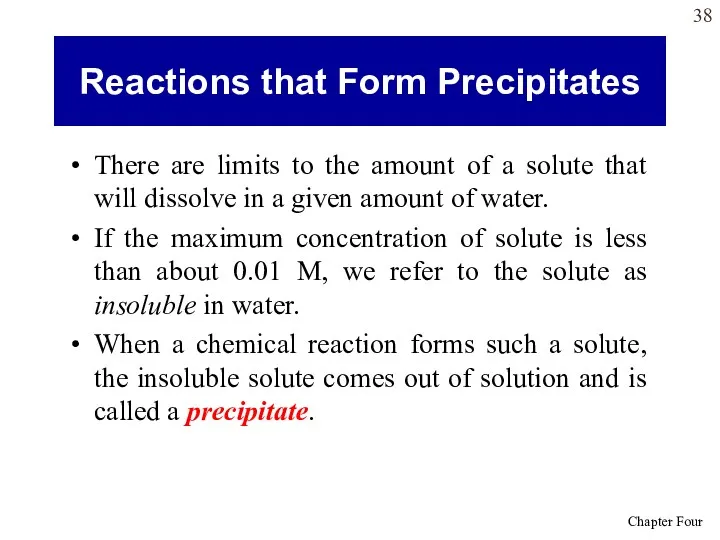
- 38. There are limits to the amount of a solute that will dissolve in a given amount
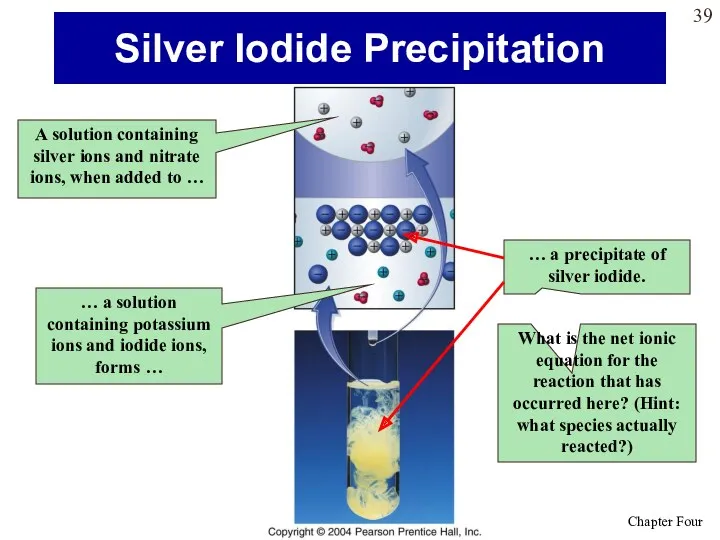
- 39. Silver Iodide Precipitation A solution containing silver ions and nitrate ions, when added to … …
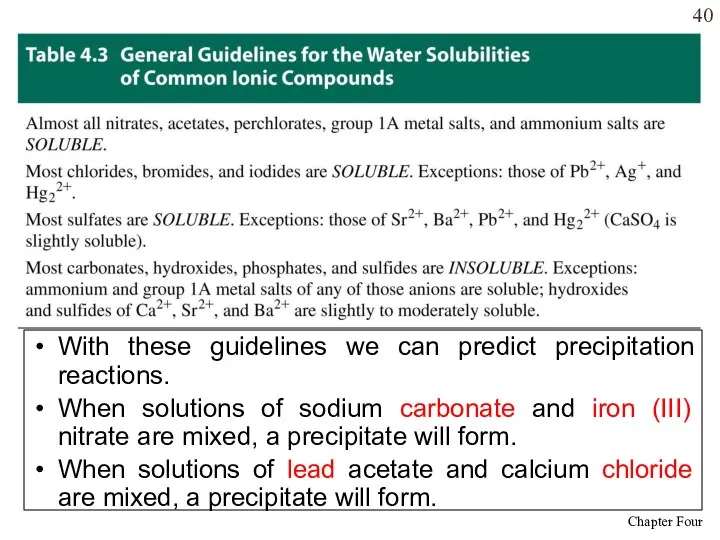
- 40. With these guidelines we can predict precipitation reactions. When solutions of sodium carbonate and iron (III)
- 42. Скачать презентацию





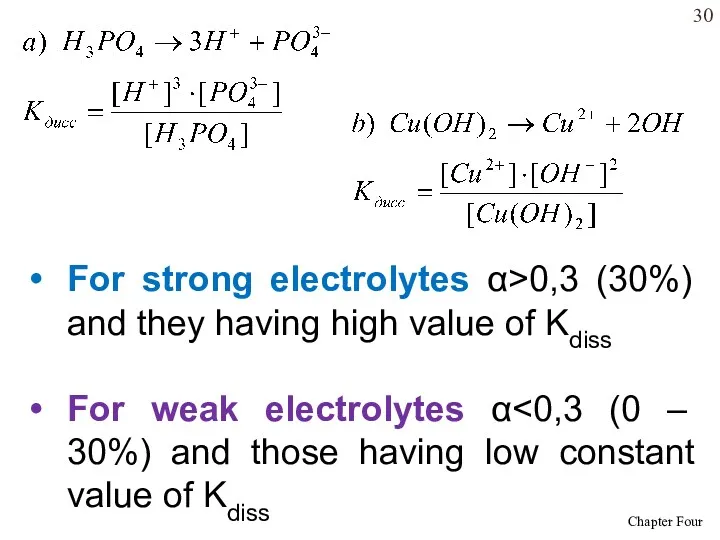
 Химия и косметика
Химия и косметика Петрология. Классификации магматических горных пород
Петрология. Классификации магматических горных пород Классы неорганических веществ
Классы неорганических веществ Потенциометрия
Потенциометрия Альдегіди і кетони аліфатичного ряду. Альдегіди і кетони ароматичного ряду
Альдегіди і кетони аліфатичного ряду. Альдегіди і кетони ароматичного ряду Аммиак
Аммиак Марганец и хром
Марганец и хром Crystal structure and surface phase composition of palladium oxides thin films for gas sensors
Crystal structure and surface phase composition of palladium oxides thin films for gas sensors Понятие о СМС и моющем процессе
Понятие о СМС и моющем процессе Щелочные металлы
Щелочные металлы Получение порошков автоклавным осаждением
Получение порошков автоклавным осаждением Растворы. Роль растворов в природе
Растворы. Роль растворов в природе Переработка газа. Первичная переработка нефти. Лекция 9
Переработка газа. Первичная переработка нефти. Лекция 9 Rhodium
Rhodium Атомы и молекулы. Простые и сложные вещества (8 класс)
Атомы и молекулы. Простые и сложные вещества (8 класс) Чистые вещества и смеси. Состав смесей. Разделение смесей
Чистые вещества и смеси. Состав смесей. Разделение смесей Химическая промышленность
Химическая промышленность 150 лет теории строения органических соединений
150 лет теории строения органических соединений Химический элемент кремний
Химический элемент кремний Водородные и кислородные соединения неметаллов. Галогеноводороды. Соляная кислота
Водородные и кислородные соединения неметаллов. Галогеноводороды. Соляная кислота Углеводороды ациклические, циклические
Углеводороды ациклические, циклические Массообменные процессы
Массообменные процессы Ионные уравнения
Ионные уравнения Строение и химические свойства кислот
Строение и химические свойства кислот Алюминий AL- химический элемент
Алюминий AL- химический элемент Материаловедение и технология обработки материалов
Материаловедение и технология обработки материалов Окислительно-восстановительные реакции. Лабораторная работа
Окислительно-восстановительные реакции. Лабораторная работа Кислоты НСL, H2 O, H2 CO3
Кислоты НСL, H2 O, H2 CO3