Crystal structure and surface phase composition of palladium oxides thin films for gas sensors презентация
Содержание
- 2. In presentation I shall focus on ten major issues: 1. Introduction – The Toxicity of Ozone
- 3. Because of health problems and noxious effects on vegetation caused by atmospheric pollution, air quality control
- 4. WHO and US EPA have established that three out of six common air pollutants (also called
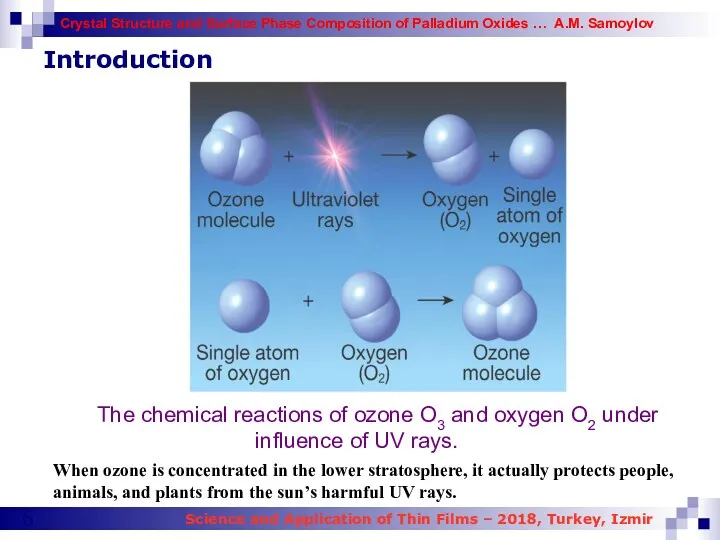
- 5. It is well known the expression that ozone gas is like a double edged sword. Most
- 6. Introduction When ozone is concentrated in the lower stratosphere, it actually protects people, animals, and plants
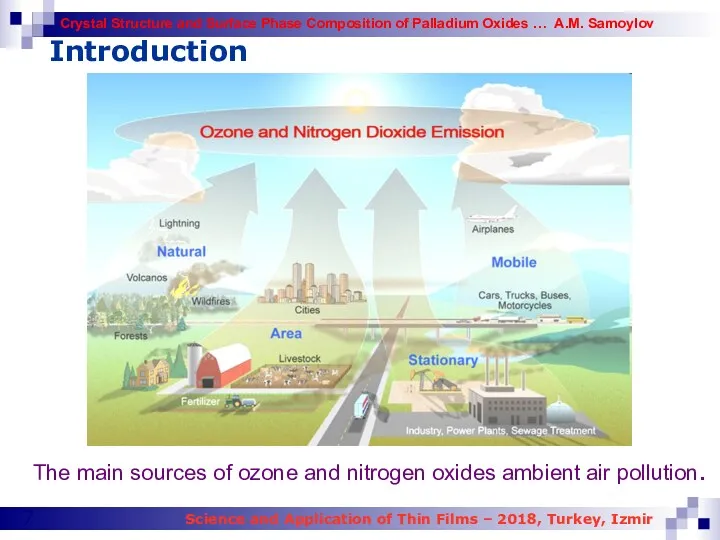
- 7. Introduction The main sources of ozone and nitrogen oxides ambient air pollution.
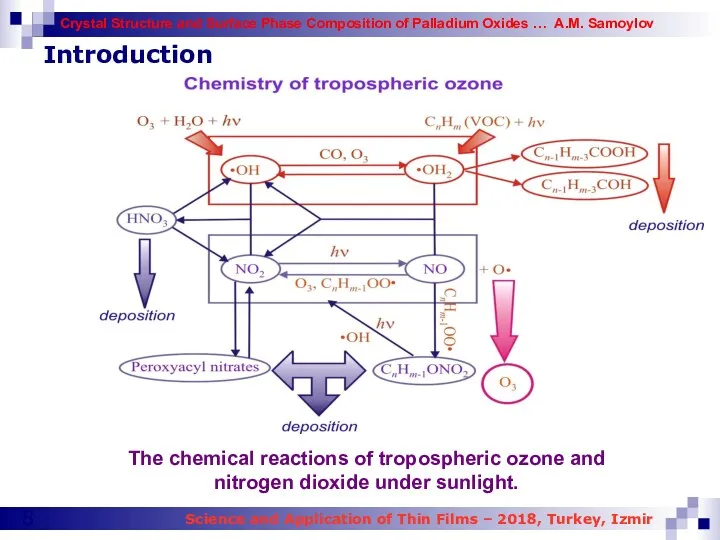
- 8. Introduction The chemical reactions of tropospheric ozone and nitrogen dioxide under sunlight.
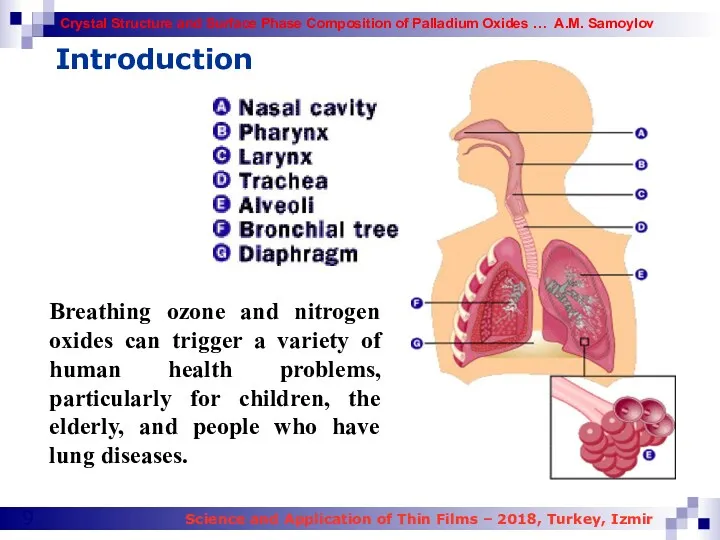
- 9. Introduction Breathing ozone and nitrogen oxides can trigger a variety of human health problems, particularly for
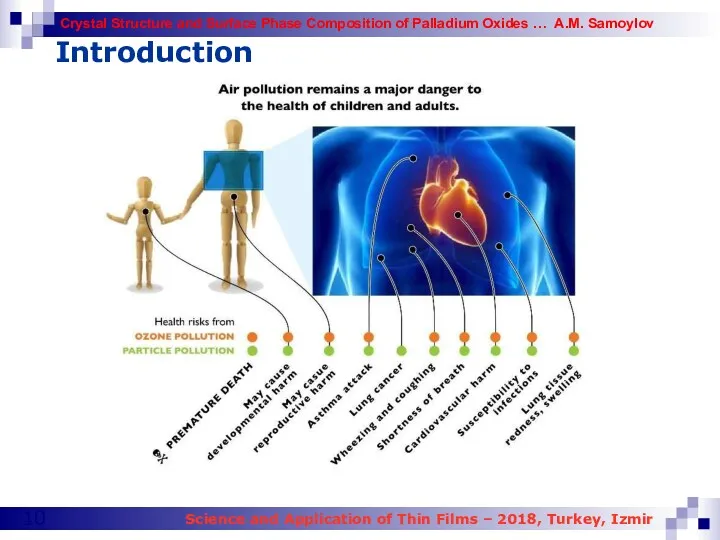
- 10. Introduction
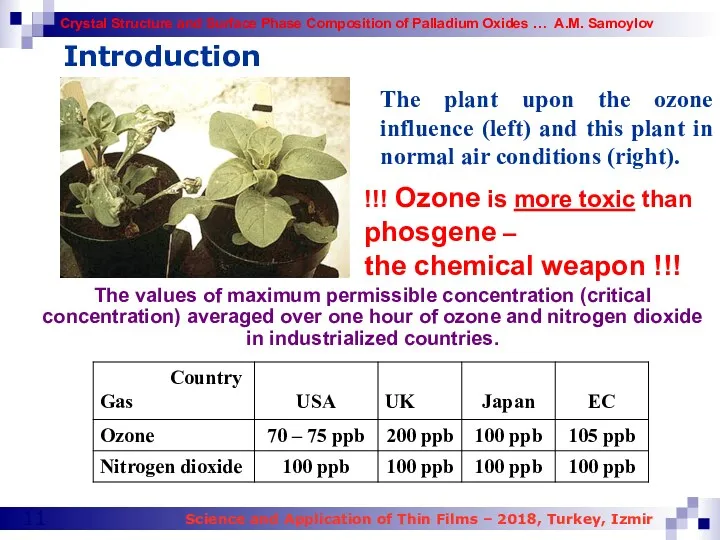
- 11. Introduction The plant upon the ozone influence (left) and this plant in normal air conditions (right).
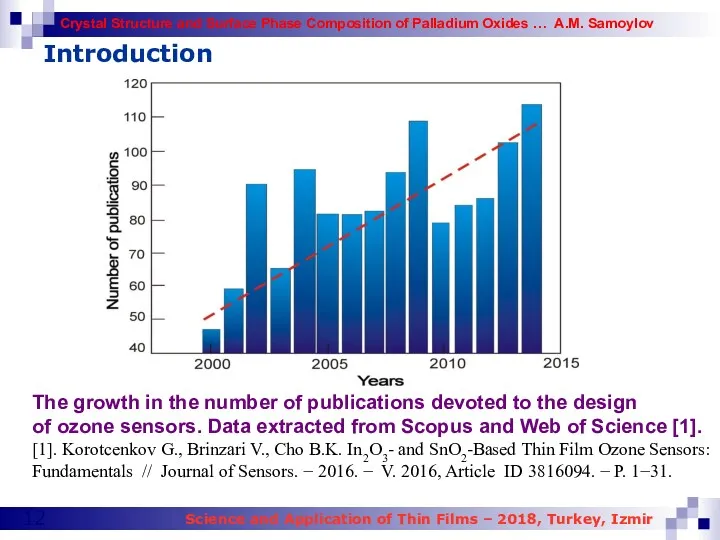
- 12. Introduction The growth in the number of publications devoted to the design of ozone sensors. Data
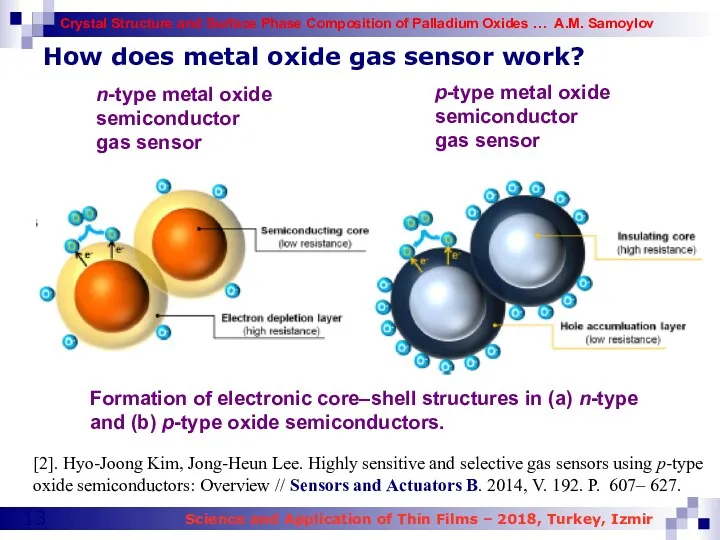
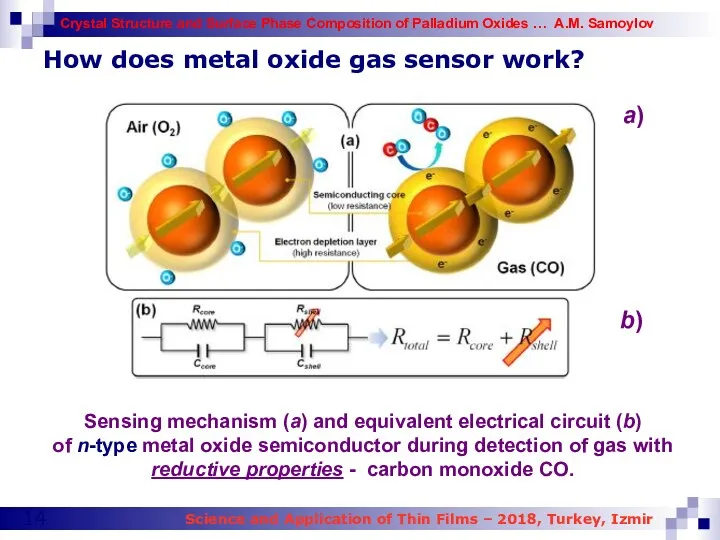
- 13. How does metal oxide gas sensor work? n-type metal oxide semiconductor gas sensor Formation of electronic
- 14. How does metal oxide gas sensor work? Sensing mechanism (a) and equivalent electrical circuit (b) of
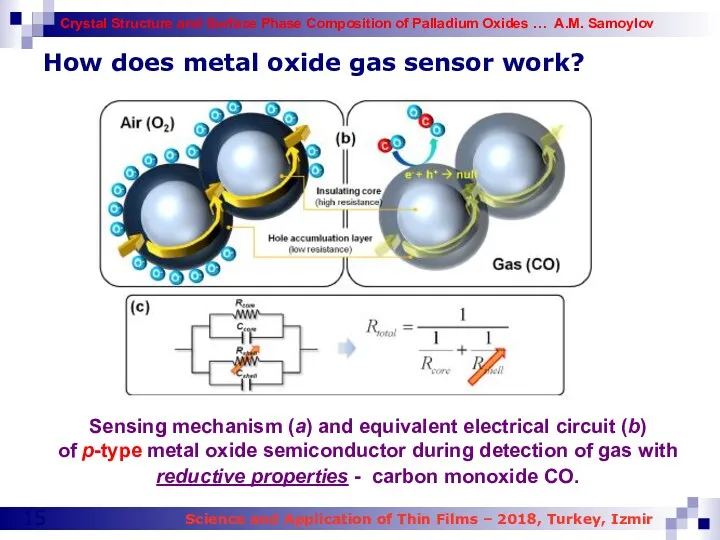
- 15. How does metal oxide gas sensor work? Sensing mechanism (a) and equivalent electrical circuit (b) of
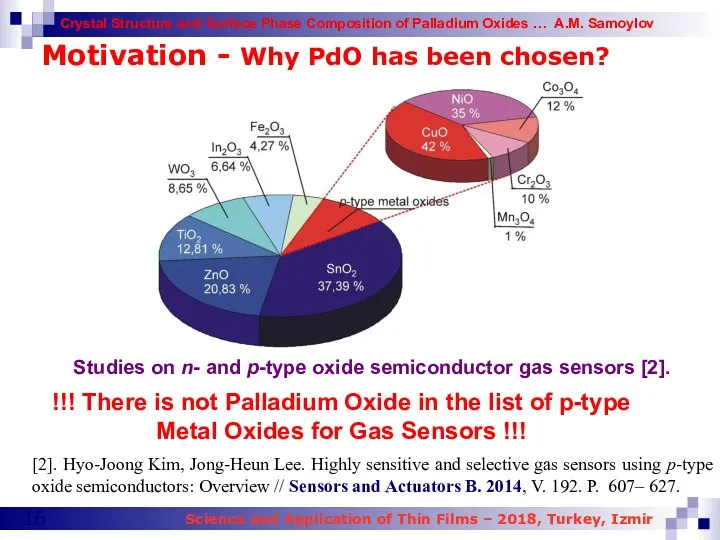
- 16. Motivation - Why PdO has been chosen? Studies on n- and p-type oxide semiconductor gas sensors
- 17. Motivation - Why PdO has been chosen? In the end of 2015 for the first time
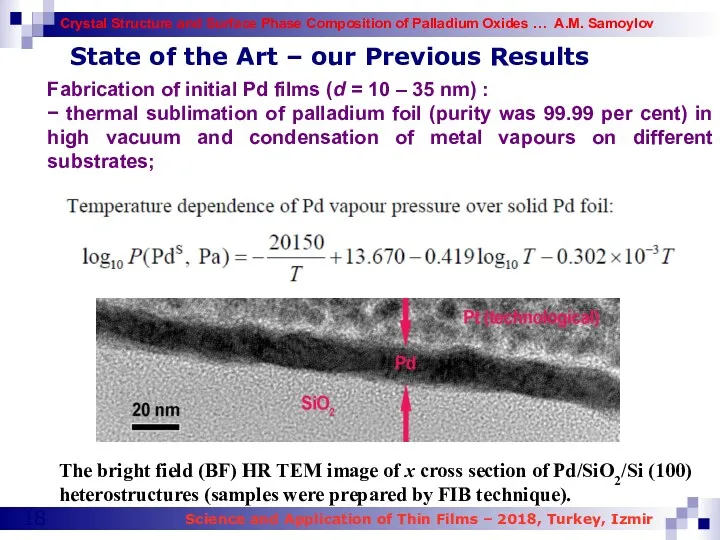
- 18. The bright field (BF) HR TEM image of x cross section of Pd/SiO2/Si (100) heterostructures (samples
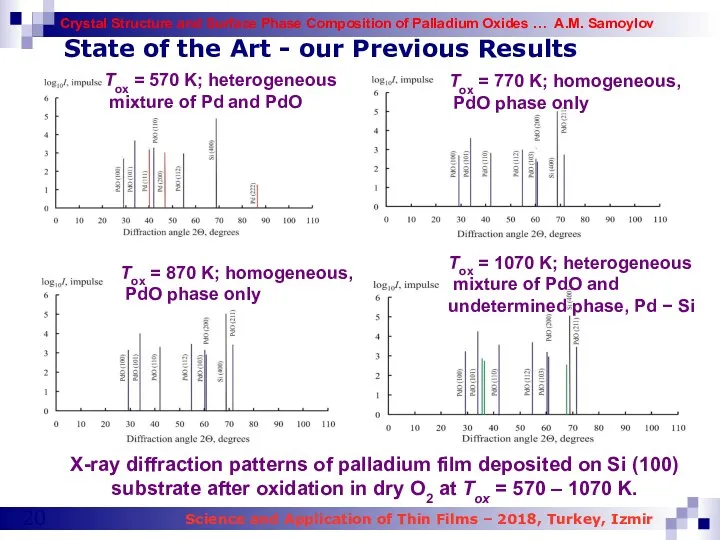
- 19. State of the Art - our Previous Results XRD (1) and THEED patterns (2 a) of
- 20. State of the Art - our Previous Results X-ray diffraction patterns of palladium film deposited on
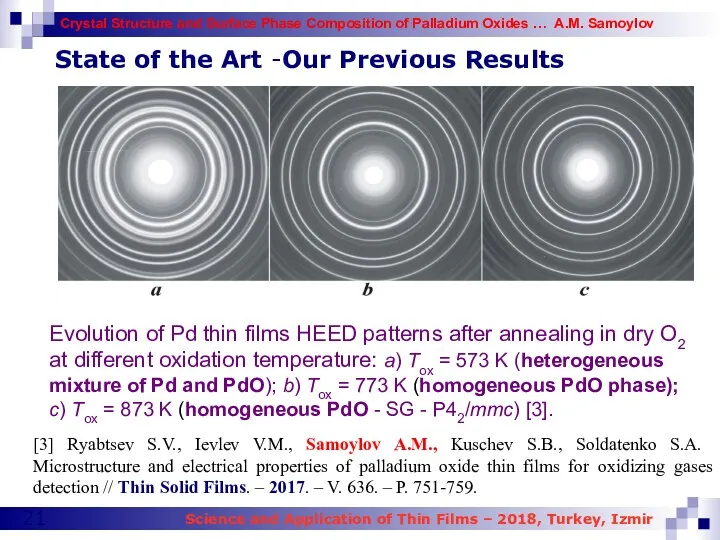
- 21. Evolution of Pd thin films HEED patterns after annealing in dry O2 at different oxidation temperature:
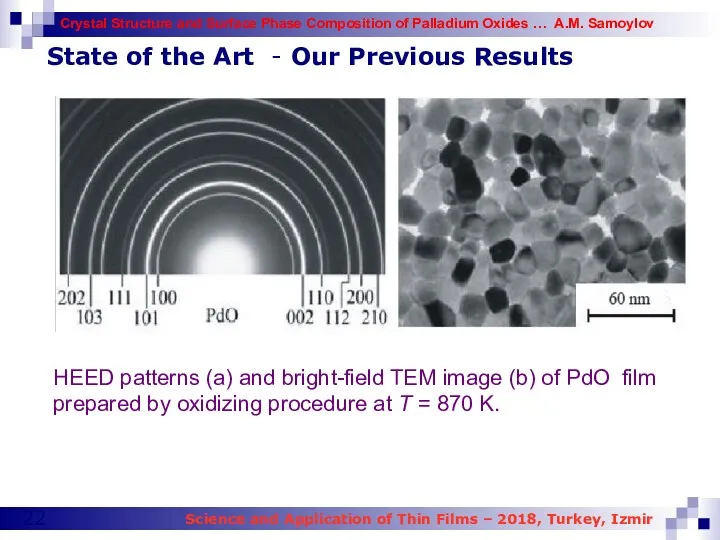
- 22. HEED patterns (a) and bright-field TEM image (b) of PdO film prepared by oxidizing procedure at
- 23. State of the Art - Our Previous Results Temperature dependence of electromotive force Eemf (a) and
- 24. State of the Art - Our Previous Results Transmission spectrum of Pd films after oxidizing at
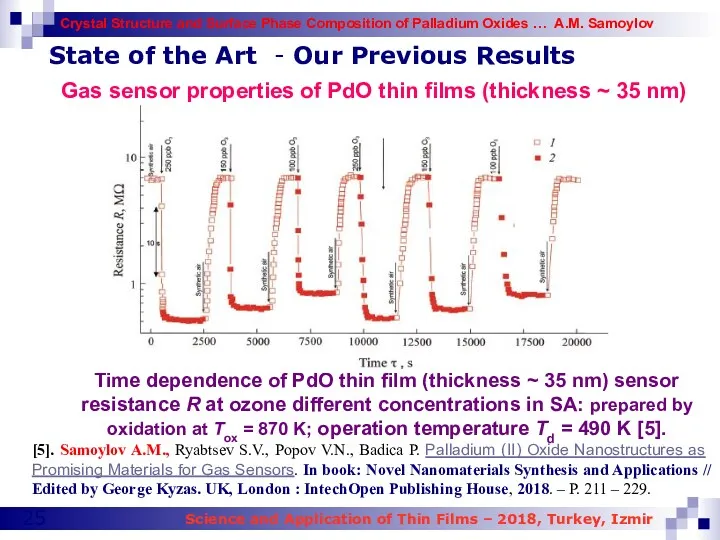
- 25. State of the Art - Our Previous Results Time dependence of PdO thin film (thickness ~
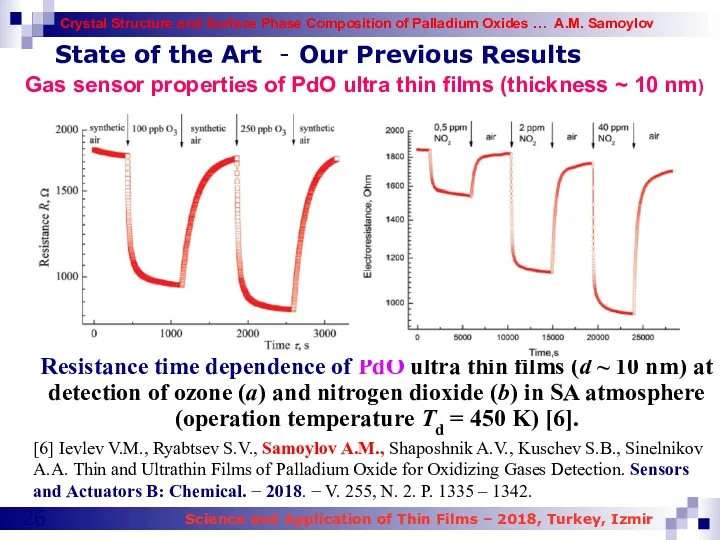
- 26. State of the Art - Our Previous Results Resistance time dependence of PdO ultra thin films
- 27. State of the Art - Our Previous Results Gas sensor properties of PdO thin films (thickness
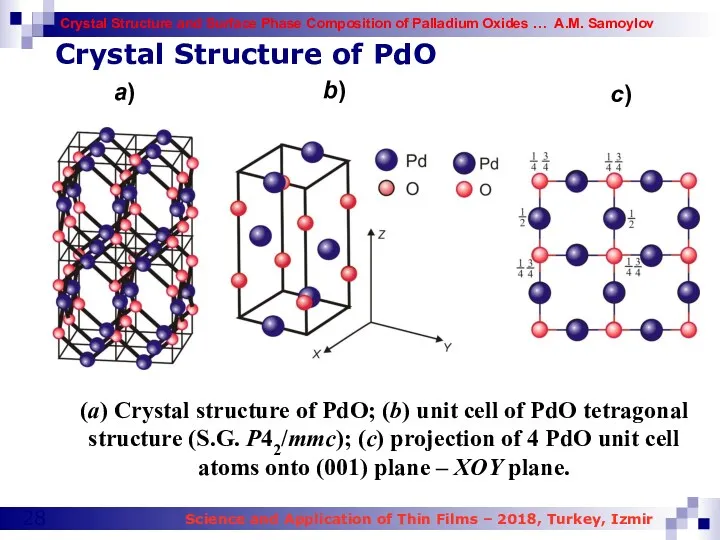
- 28. (a) Crystal structure of PdO; (b) unit cell of PdO tetragonal structure (S.G. P42/mmc); (c) projection
- 29. Problem of PdO film Nonstoichiometry Taking into account the nonstoichiometry of PdO caused by O atom
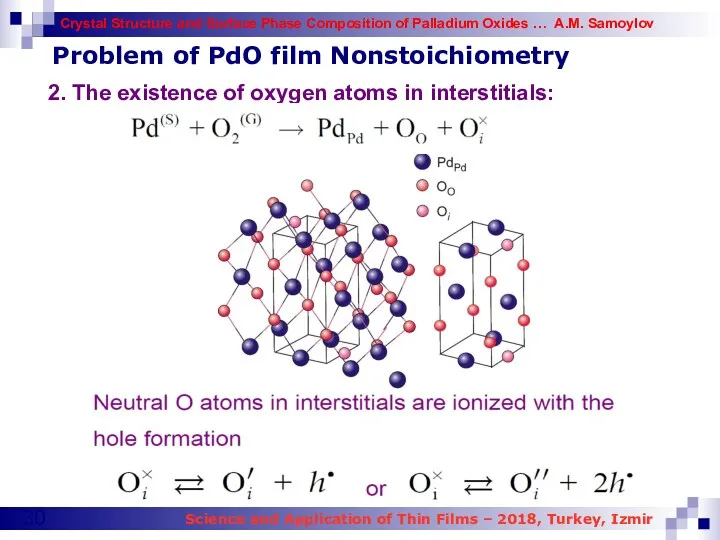
- 30. Problem of PdO film Nonstoichiometry 2. The existence of oxygen atoms in interstitials:
- 31. The Main Purpose of this Study The main purpose of this work is the complex study
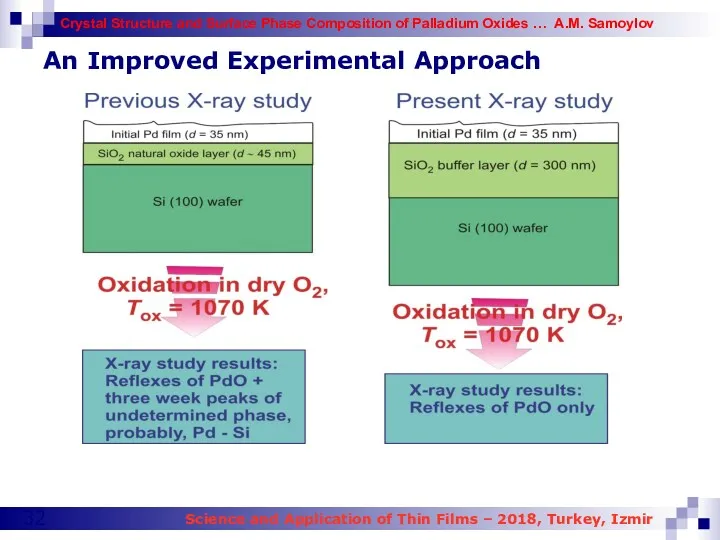
- 32. An Improved Experimental Approach
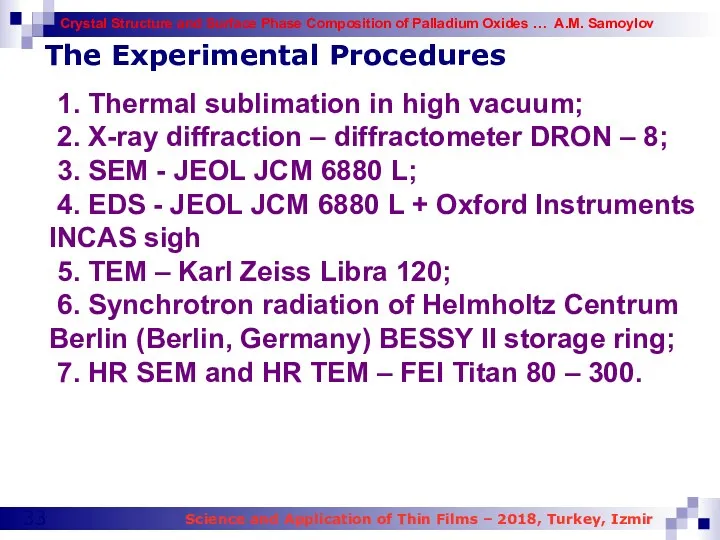
- 33. The Experimental Procedures 1. Thermal sublimation in high vacuum; 2. X-ray diffraction – diffractometer DRON –
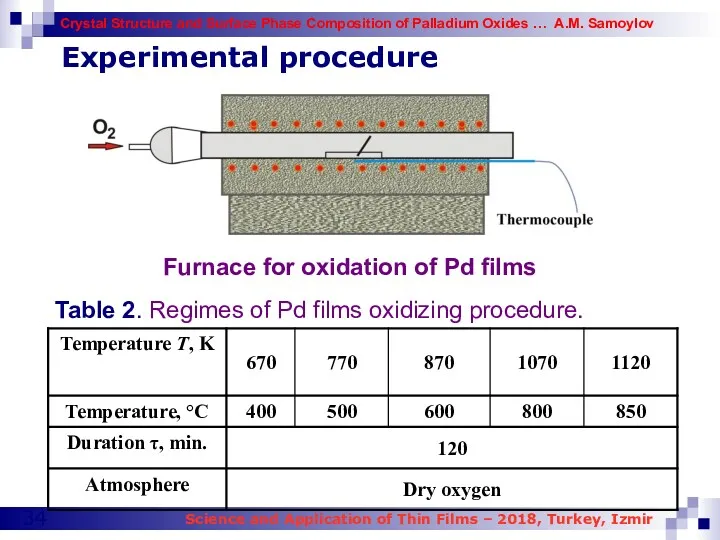
- 34. Experimental procedure Furnace for oxidation of Pd films Table 2. Regimes of Pd films oxidizing procedure.
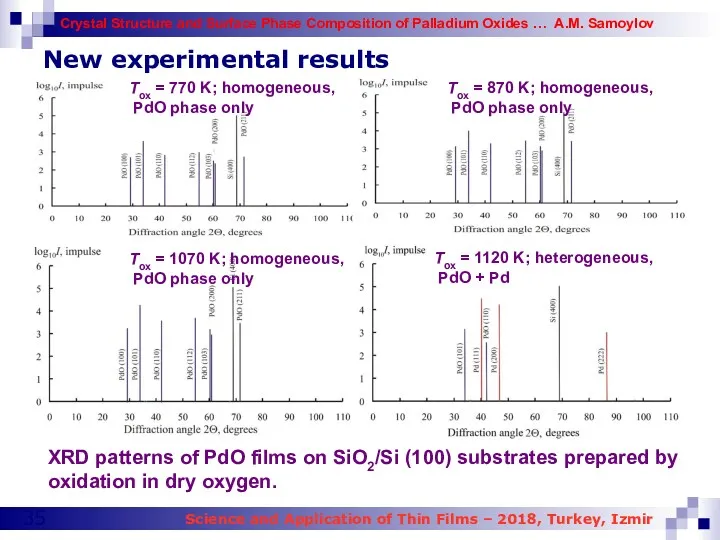
- 35. New experimental results XRD patterns of PdO films on SiO2/Si (100) substrates prepared by oxidation in
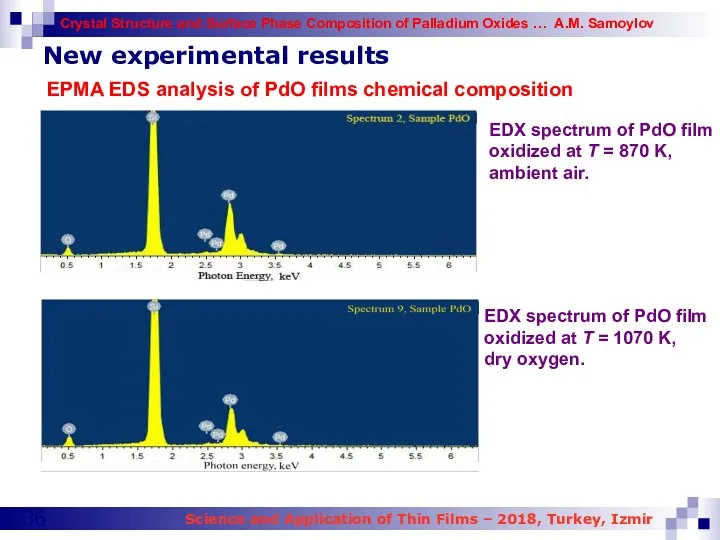
- 36. New experimental results EPMA EDS analysis of PdO films chemical composition EDX spectrum of PdO film
- 37. New experimental results EPMA EDS analysis of PdO films chemical composition Dependence of n(O)/n(Pd) ratio upon
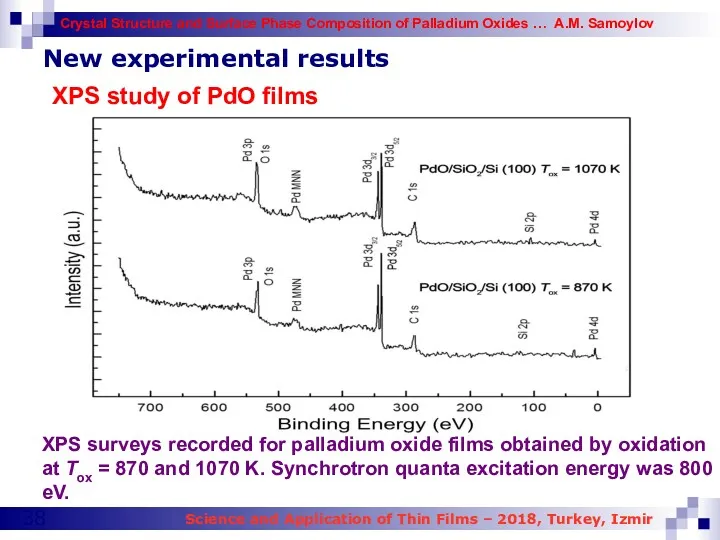
- 38. New experimental results XPS study of PdO films XPS surveys recorded for palladium oxide films obtained
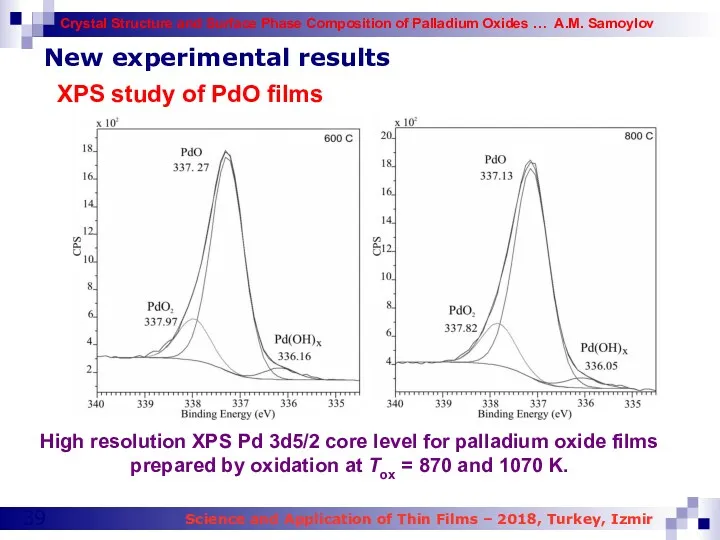
- 39. New experimental results XPS study of PdO films High resolution XPS Pd 3d5/2 core level for
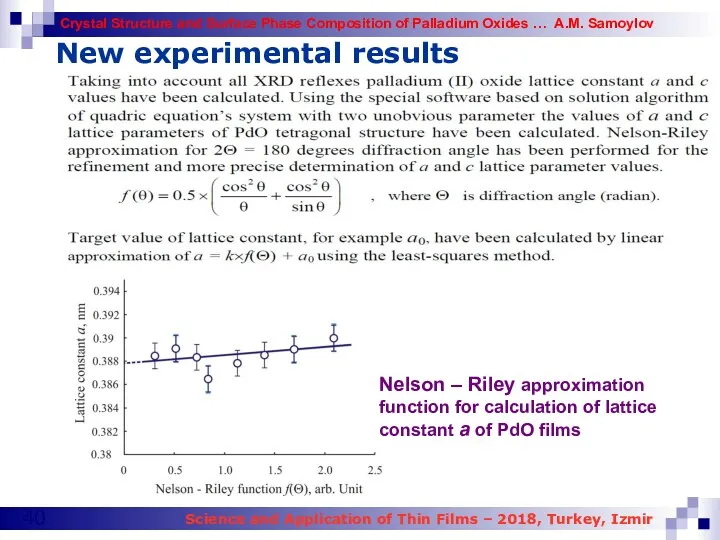
- 40. New experimental results Nelson – Riley approximation function for calculation of lattice constant a of PdO
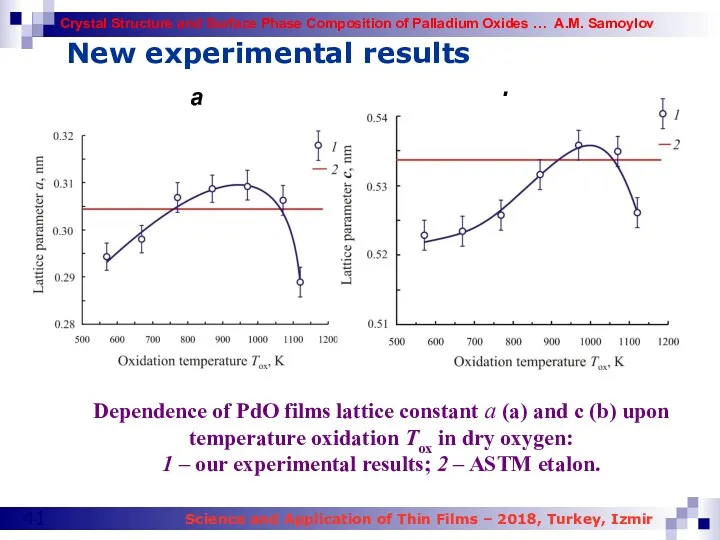
- 41. New experimental results Dependence of PdO films lattice constant a (a) and c (b) upon temperature
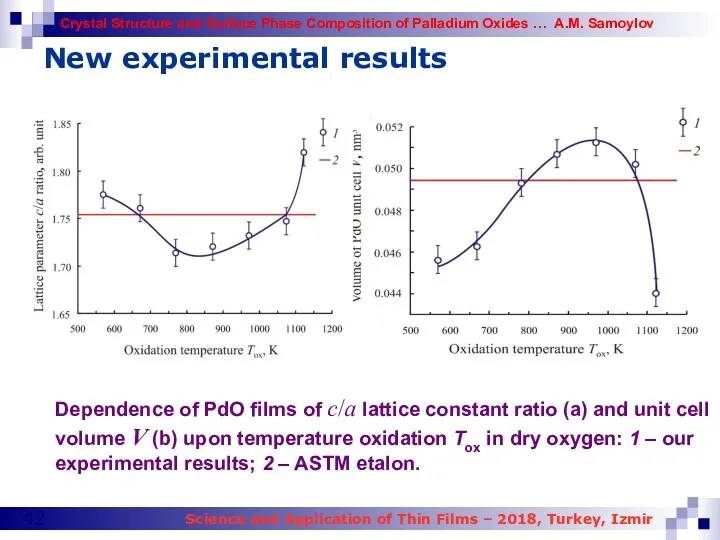
- 42. New experimental results Dependence of PdO films of c/a lattice constant ratio (a) and unit cell
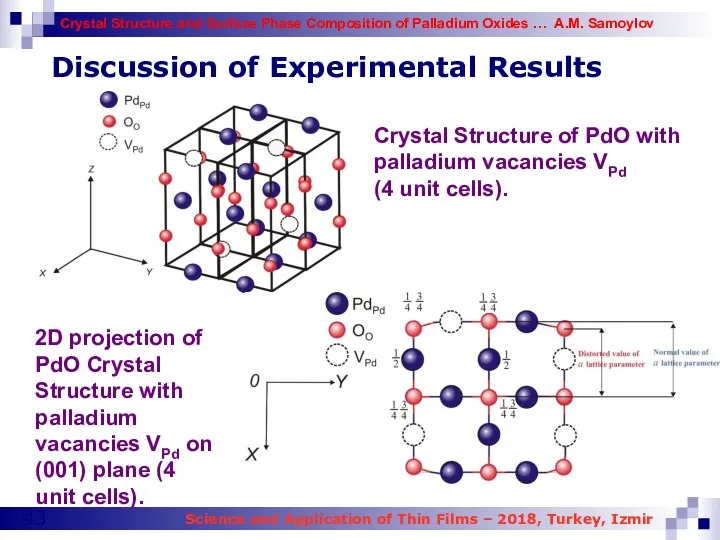
- 43. Discussion of Experimental Results Crystal Structure of PdO with palladium vacancies VPd (4 unit cells). 2D
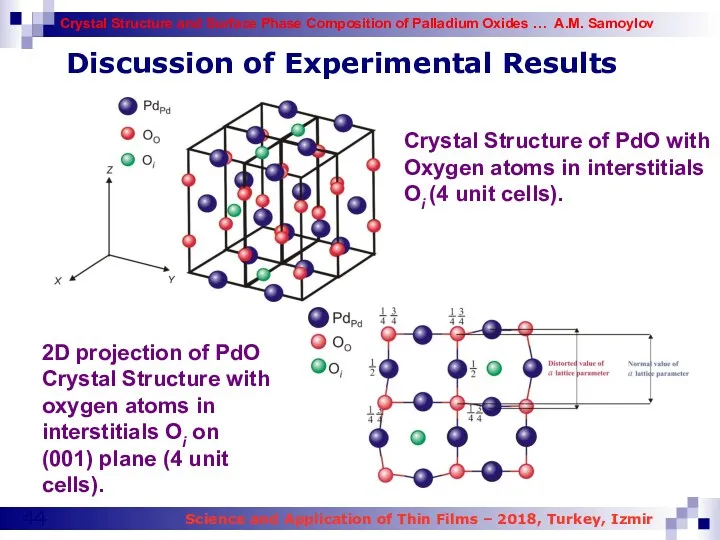
- 44. Discussion of Experimental Results Crystal Structure of PdO with Oxygen atoms in interstitials Oi (4 unit
- 45. Summary and Conclusion It is necessary to note that results obtained in this work are the
- 46. Future Study The sensitivity of PdO based gas sensor can be improved by some ways: The
- 47. THANK YOU FOR YOUR ATTENTION !
- 48. If You have any questions or suggestion You can find me: Phone: +7 951 552 7564
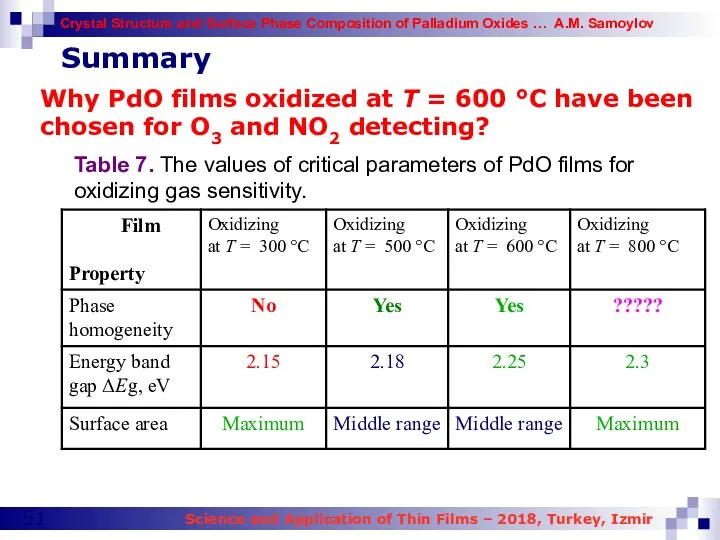
- 49. Future study Why PdO films oxidized at T = 600 °C have been chosen for O3
- 50. Summary and Conclusion
- 51. Why PdO films oxidized at T = 600 °C have been chosen for O3 and NO2
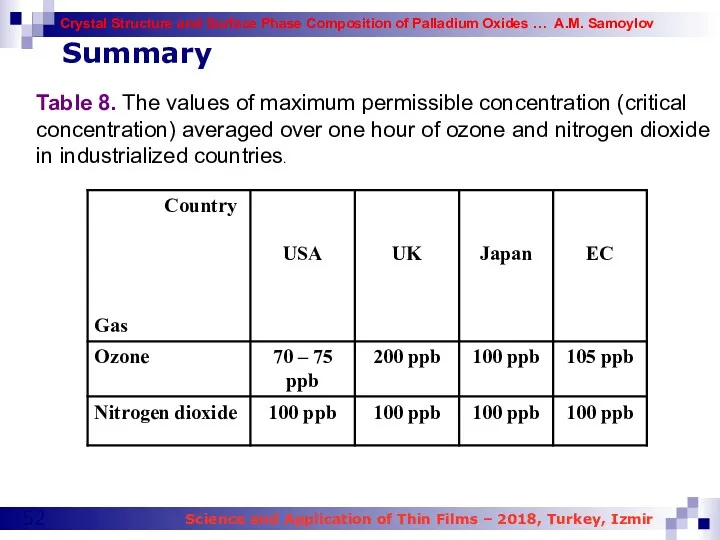
- 52. Table 8. The values of maximum permissible concentration (critical concentration) averaged over one hour of ozone
- 53. At fabrication of gas sensors usage of PdO1±x thin films has some advantages in comparison with
- 54. Work for Future Because we started PdO investigation in September, 2015, today we have questions more
- 55. Authors are thankful to the Russian Scientific Foundation (RSF) for financial support of this activity, project
- 57. Скачать презентацию


















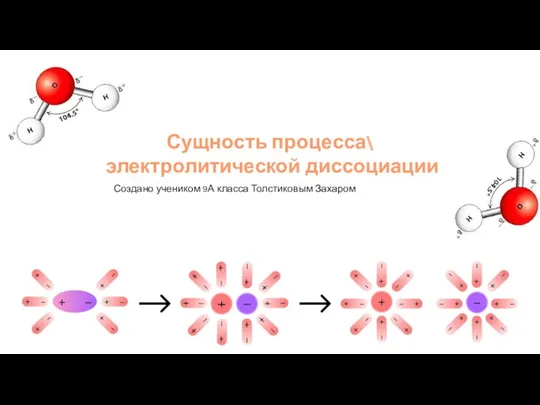 Сущность процесса электролитической диссоциации
Сущность процесса электролитической диссоциации Типы химических реакций. Тепловой эффект (11 класс)
Типы химических реакций. Тепловой эффект (11 класс) Азотсодержащие гетероциклические соединения
Азотсодержащие гетероциклические соединения Crystal defects
Crystal defects Классификация минералов по химическому принципу. Кварц, магнетит
Классификация минералов по химическому принципу. Кварц, магнетит Інгібіювання та регуляція ензимів
Інгібіювання та регуляція ензимів Аммиак. Состав вещества
Аммиак. Состав вещества Le trasformazioni fisiche della materia. Tema 3
Le trasformazioni fisiche della materia. Tema 3 Введение в химию
Введение в химию Обмен нуклеотидов
Обмен нуклеотидов Основні технологічні процеси очистки води. Знезараження води. Знезараження води хлором
Основні технологічні процеси очистки води. Знезараження води. Знезараження води хлором Возраст в геологии
Возраст в геологии Соединения щелочных металлов
Соединения щелочных металлов Оксиди: поняття, склад і назви, фізичні властивості, поширеність у природі, використання
Оксиди: поняття, склад і назви, фізичні властивості, поширеність у природі, використання Аммиак. Образование молекулы аммиака
Аммиак. Образование молекулы аммиака Химическая связь. (Лекция 4, 5)
Химическая связь. (Лекция 4, 5) Гетерофункциональные производные бензольного ряда как лекарственные средства
Гетерофункциональные производные бензольного ряда как лекарственные средства Кислородные соединения азота
Кислородные соединения азота Закон сохранения массы веществ. Химические уравнения
Закон сохранения массы веществ. Химические уравнения Метаболизм кетоновых тел. Метаболизм холестерина
Метаболизм кетоновых тел. Метаболизм холестерина Предельные углеводороды
Предельные углеводороды Теория электролитической диссоциации (ТЭД)
Теория электролитической диссоциации (ТЭД) Массообменные процессы
Массообменные процессы Снежинки. Рождение снежинки
Снежинки. Рождение снежинки Аномальные свойства воды
Аномальные свойства воды Алмастырылмайтын аминқышқылды алу биотехнологиясы
Алмастырылмайтын аминқышқылды алу биотехнологиясы Промежуточные фазы в металлических сплавах
Промежуточные фазы в металлических сплавах Моющие и чистящие средства
Моющие и чистящие средства