Содержание
- 2. Contents 1. Understanding of Rate of Reaction 2. Factors Affecting Rate of Reaction 3. Collision Theory
- 3. Chemical reactions require varying lengths of time for completion, depending on the characteristics of the reactants
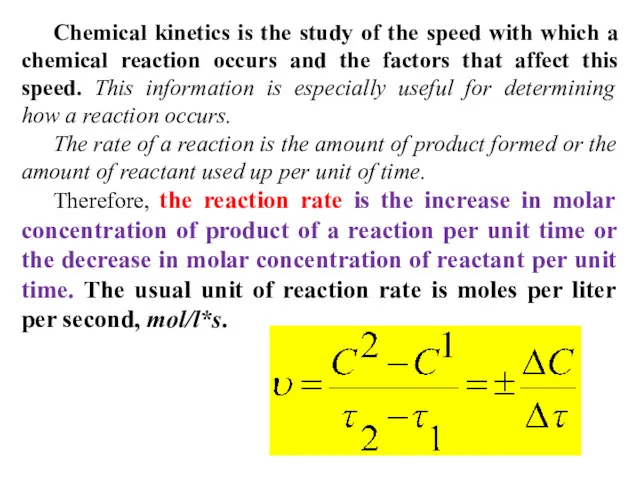
- 4. Chemical kinetics is the study of the speed with which a chemical reaction occurs and the
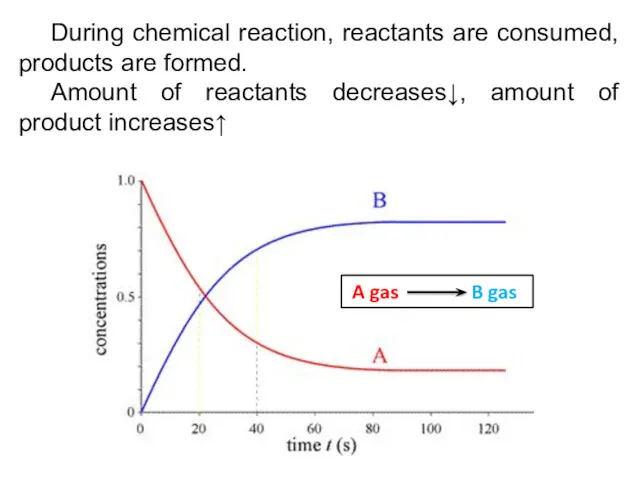
- 5. During chemical reaction, reactants are consumed, products are formed. Amount of reactants decreases↓, amount of product
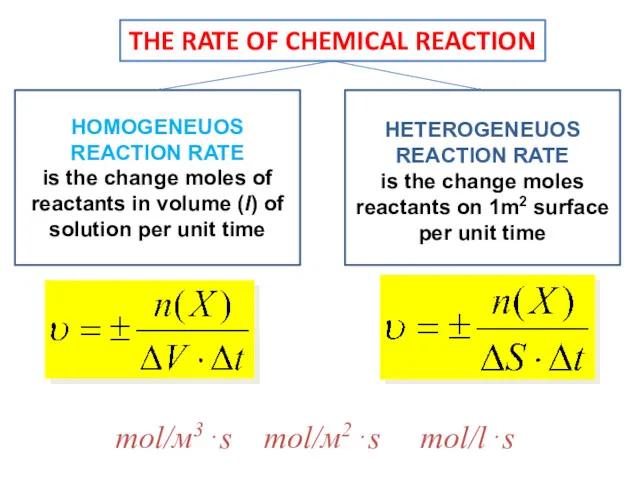
- 6. mol/м3⋅s mol/м2⋅s mol/l⋅s THE RATE OF CHEMICAL REACTION HOMOGENEUOS REACTION RATE is the change moles of
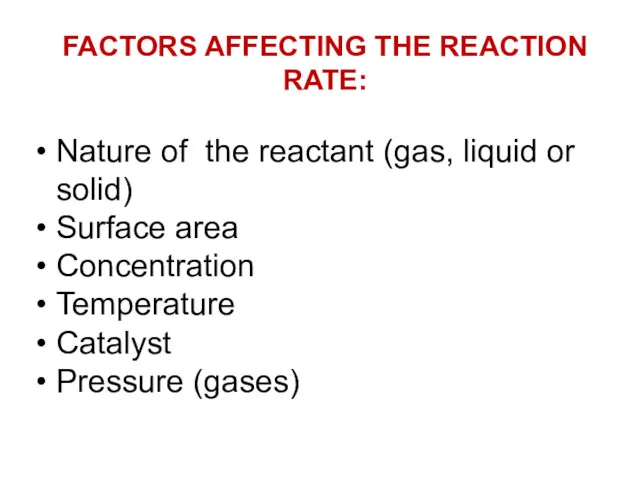
- 7. FACTORS AFFECTING THE REACTION RATE: Nature of the reactant (gas, liquid or solid) Surface area Concentration
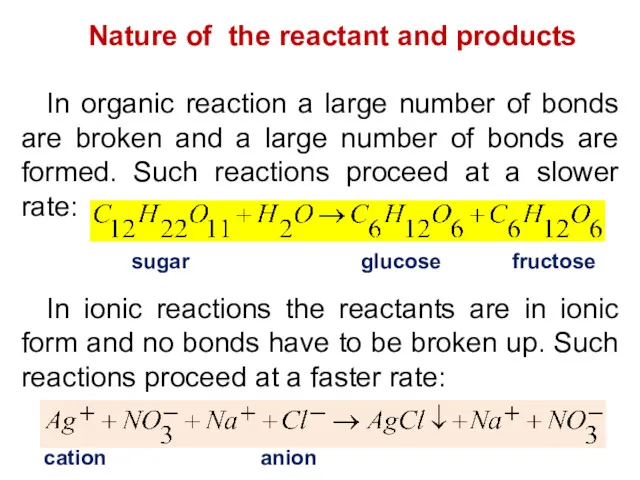
- 8. Nature of the reactant and products In organic reaction a large number of bonds are broken
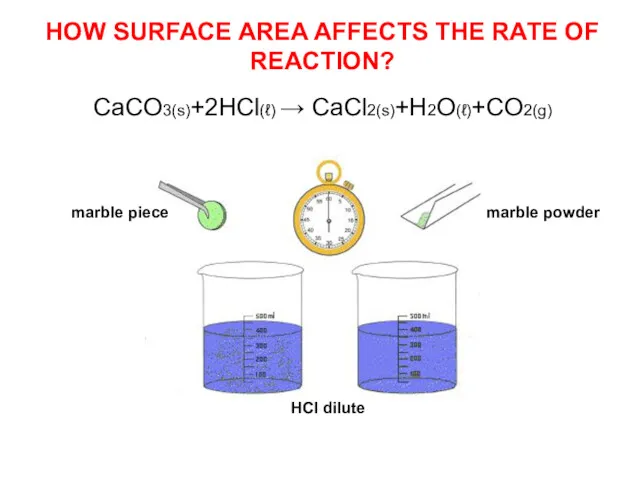
- 9. HOW SURFACE AREA AFFECTS THE RATE OF REACTION? CaCO3(s)+2HCl(ℓ) → CaCl2(s)+H2O(ℓ)+CO2(g) marble piece marble powder HCl
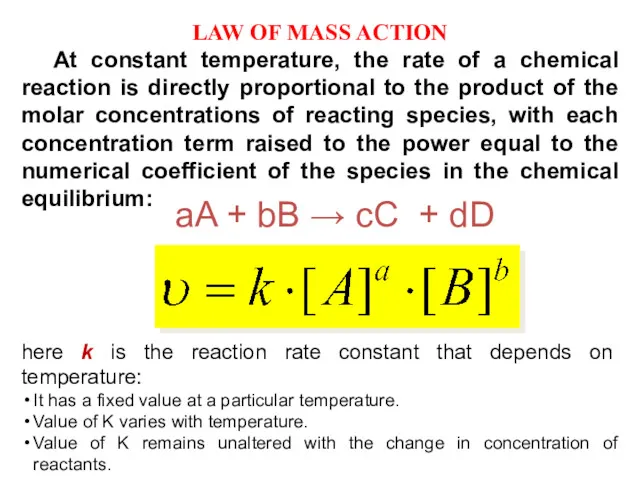
- 10. LAW OF MASS ACTION At constant temperature, the rate of a chemical reaction is directly proportional
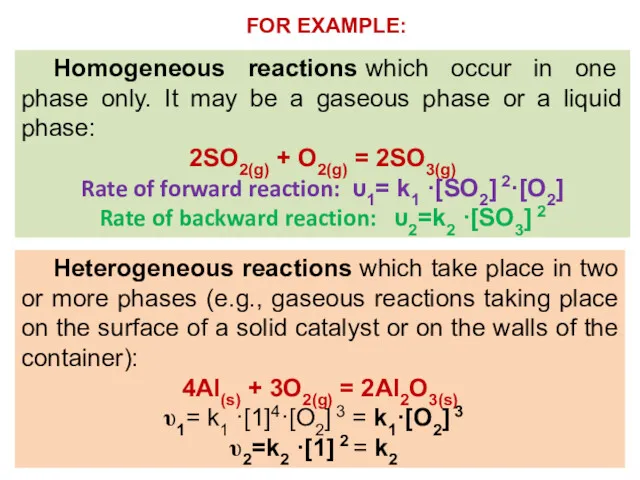
- 11. Homogeneous reactions which occur in one phase only. It may be a gaseous phase or a
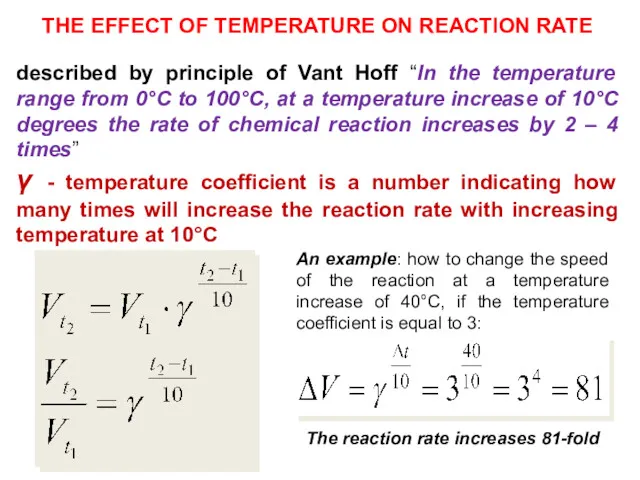
- 12. THE EFFECT OF TEMPERATURE ON REACTION RATE described by principle of Vant Hoff “In the temperature
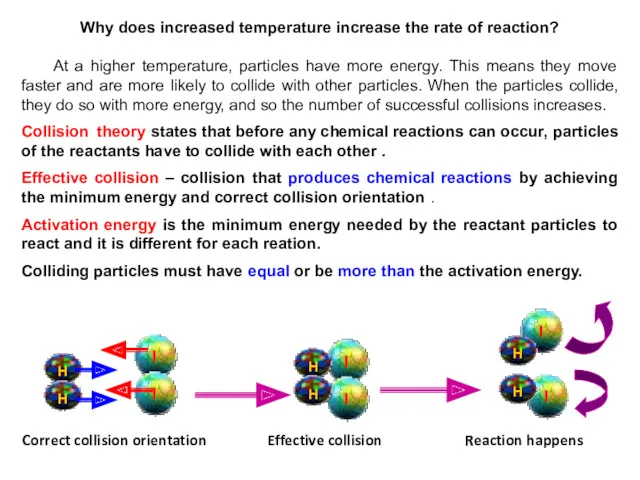
- 13. Why does increased temperature increase the rate of reaction? At a higher temperature, particles have more
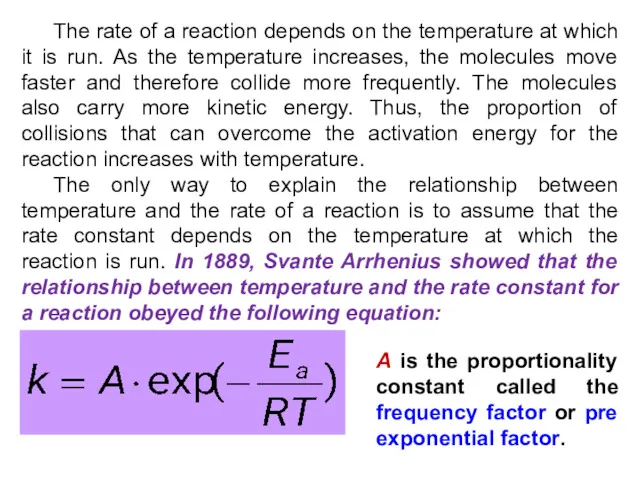
- 14. The rate of a reaction depends on the temperature at which it is run. As the
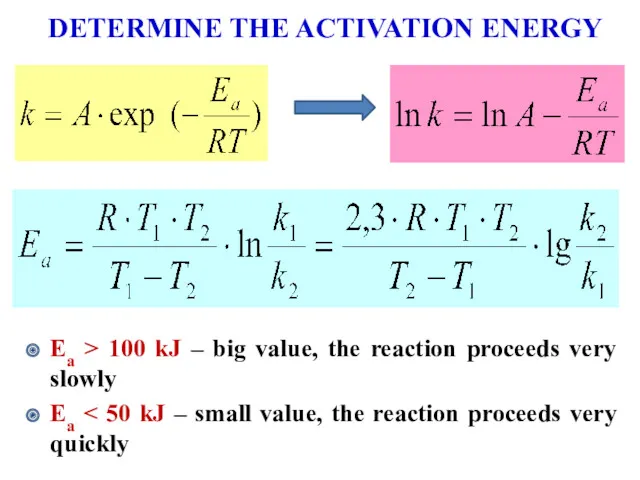
- 15. DETERMINE THE ACTIVATION ENERGY Еа > 100 kJ – big value, the reaction proceeds very slowly
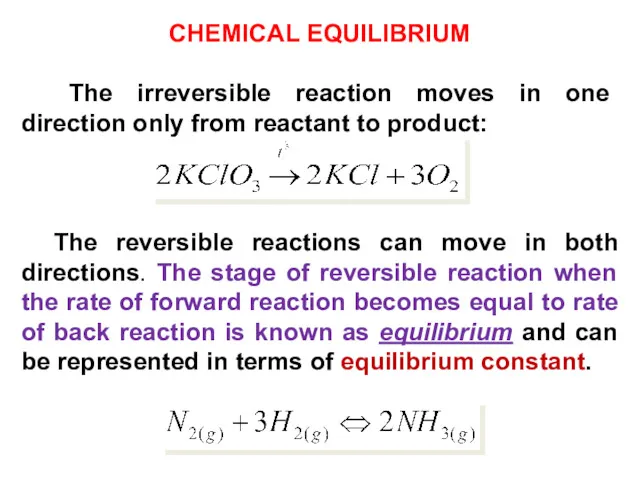
- 16. CHEMICAL EQUILIBRIUM The irreversible reaction moves in one direction only from reactant to product: The reversible
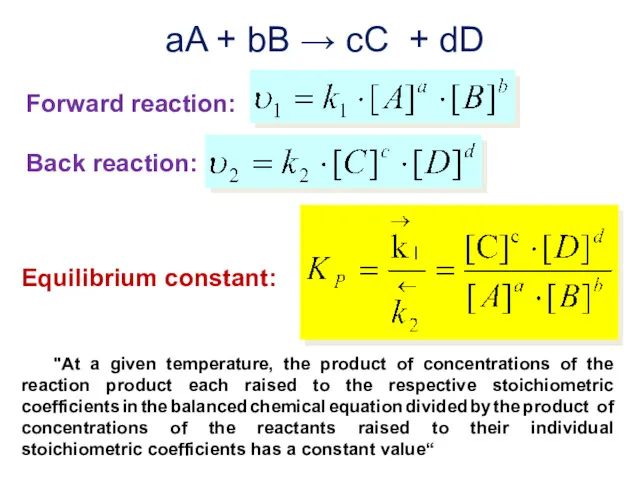
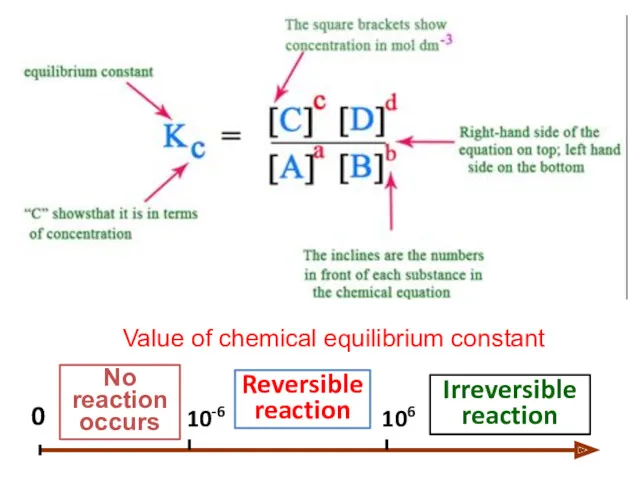
- 17. "At a given temperature, the product of concentrations of the reaction product each raised to the
- 18. 0 106 10-6 Reversible reaction Irreversible reaction No reaction occurs Value of chemical equilibrium constant
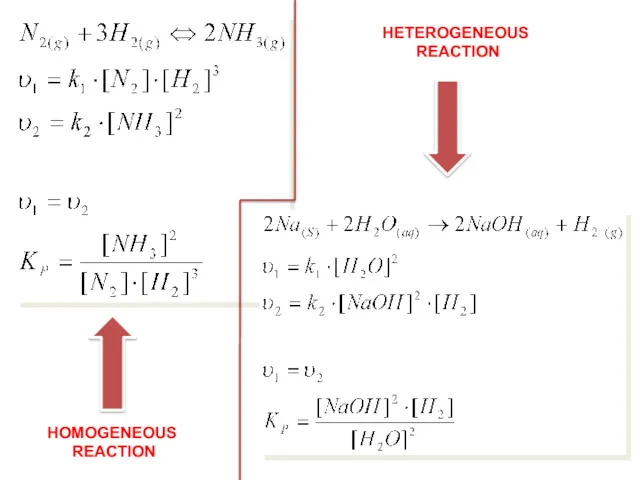
- 19. HOMOGENEOUS REACTION HETEROGENEOUS REACTION
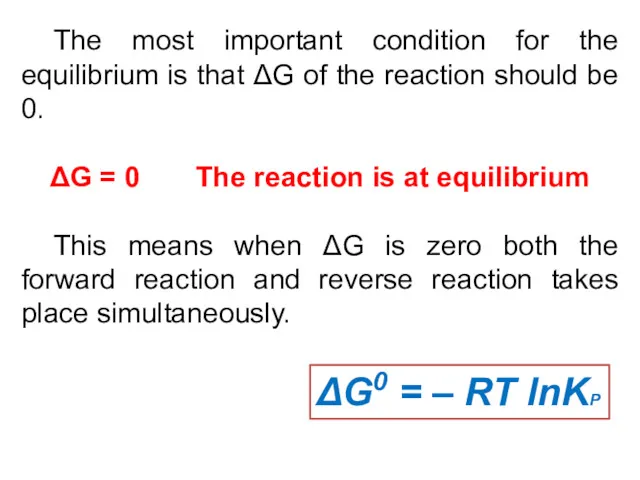
- 20. The most important condition for the equilibrium is that ΔG of the reaction should be 0.
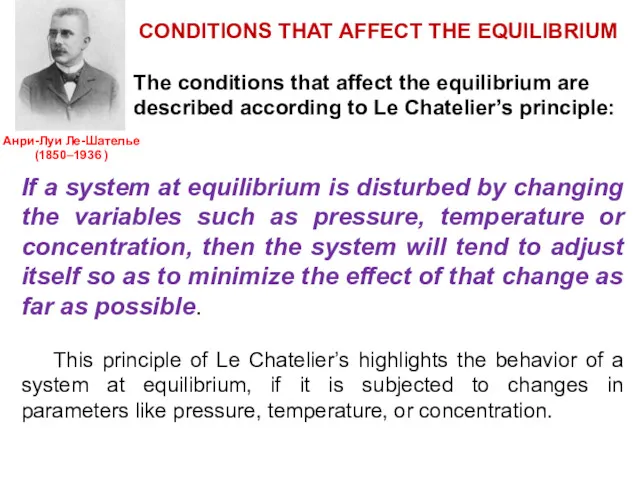
- 21. CONDITIONS THAT AFFECT THE EQUILIBRIUM The conditions that affect the equilibrium are described according to Le
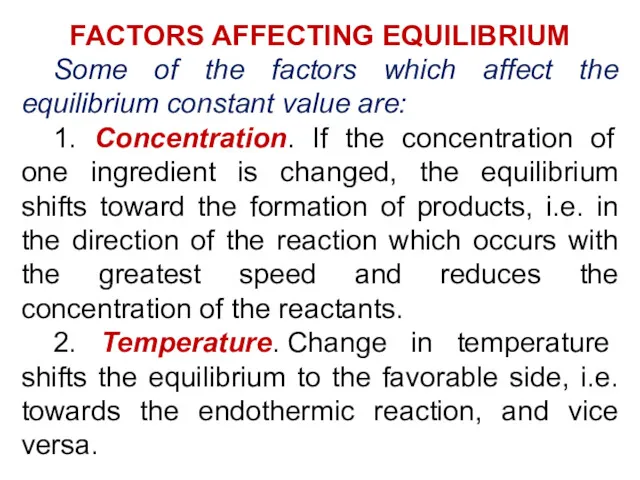
- 22. FACTORS AFFECTING EQUILIBRIUM Some of the factors which affect the equilibrium constant value are: 1. Concentration.
- 23. 3. Pressure. The pressure change affects only on those systems where in at least one of
- 24. CATALYST A catalyst will change the rate of reaction. A catalyst only changes the rate of
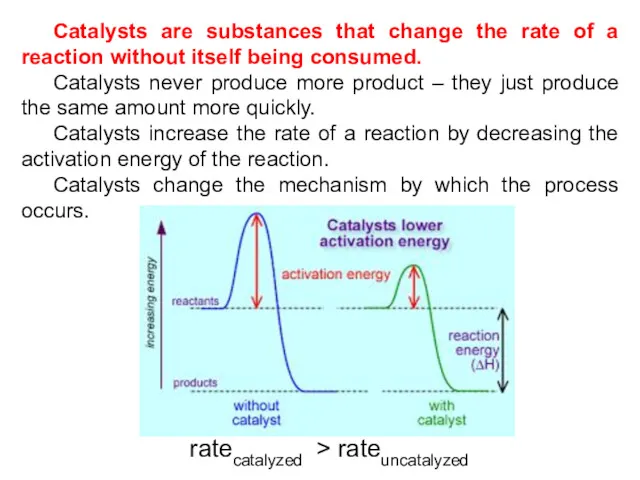
- 25. Catalysts are substances that change the rate of a reaction without itself being consumed. Catalysts never
- 26. QUIZ ME NEXT 1 What is the discipline that studies chemical reactions with respect to reaction
- 27. QUIZ ME NEXT 2 What drives chemical reactions? Electrons Physical conditions Energy Activation Energy
- 28. QUIZ ME NEXT 3 Which one of the following reactions reacts the most rapidly at room
- 30. Скачать презентацию



 Природные источники углеводородов
Природные источники углеводородов СПЛАВЫ. КОРРОЗИЯ МЕТАЛЛОВ
СПЛАВЫ. КОРРОЗИЯ МЕТАЛЛОВ Алкадиены
Алкадиены Квантовые числа
Квантовые числа Класифікація та властивості оксидів
Класифікація та властивості оксидів Положение в ПСХЭ водорода, лантаноидов, актиноидов и искусственно полученных элементов
Положение в ПСХЭ водорода, лантаноидов, актиноидов и искусственно полученных элементов Тепловой эффект химических реакций. 8 класс
Тепловой эффект химических реакций. 8 класс Основы химической термодинамики
Основы химической термодинамики 20230321_obobshchenie_po_elektroliticheskoy_dissotsiatsii
20230321_obobshchenie_po_elektroliticheskoy_dissotsiatsii Қаныққан бір атомды спитртер
Қаныққан бір атомды спитртер Спирты, фенолы, тиолы
Спирты, фенолы, тиолы Көмірсулар Қайталау сабағы
Көмірсулар Қайталау сабағы Вольфрам — химический элемент
Вольфрам — химический элемент Золото
Золото Анализ качества лекарственных веществ, определяемых методом комплексонометрии
Анализ качества лекарственных веществ, определяемых методом комплексонометрии Хімічна кінетика
Хімічна кінетика Задания С 3 для подготовки к ГИА -9 по химии
Задания С 3 для подготовки к ГИА -9 по химии Технология производства аминоальдегидных смол
Технология производства аминоальдегидных смол Закономерность изменений в строении электронных оболочек атома
Закономерность изменений в строении электронных оболочек атома Химическая промышленность России входит в авангардную тройку
Химическая промышленность России входит в авангардную тройку Физические и химические свойства алмаза. Алмазная промышленность в России
Физические и химические свойства алмаза. Алмазная промышленность в России Литология. Кремнистые породы
Литология. Кремнистые породы ЕГЭ Химия. Задание №5
ЕГЭ Химия. Задание №5 Химические свойства металлов
Химические свойства металлов Посуда, ее виды и использование
Посуда, ее виды и использование Неметаллические материалы
Неметаллические материалы Полиэтилен - термопластичный полимер этилена
Полиэтилен - термопластичный полимер этилена Основы коррозии и защиты металлов. Химическая коррозия
Основы коррозии и защиты металлов. Химическая коррозия