Содержание
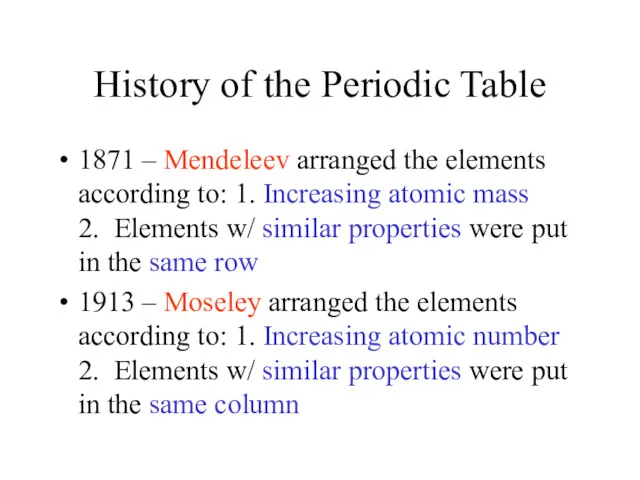
- 2. History of the Periodic Table 1871 – Mendeleev arranged the elements according to: 1. Increasing atomic
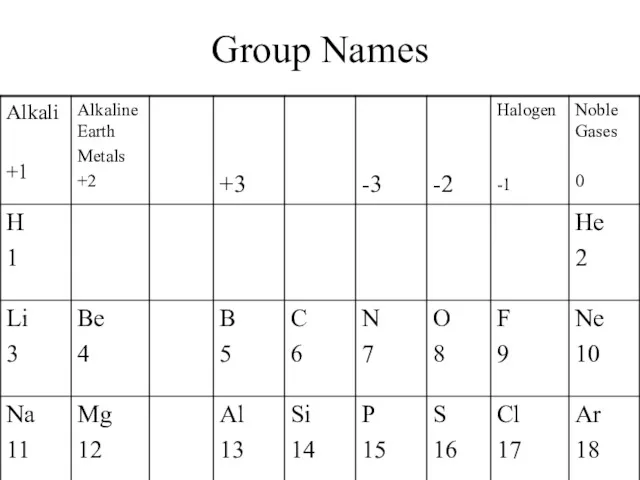
- 3. Group Names
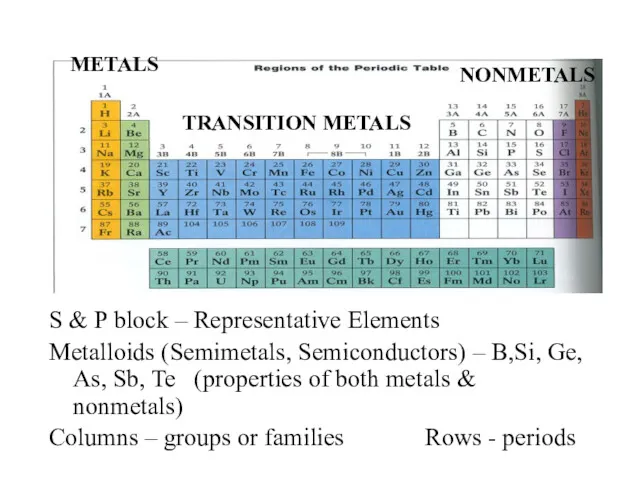
- 4. S & P block – Representative Elements Metalloids (Semimetals, Semiconductors) – B,Si, Ge, As, Sb, Te
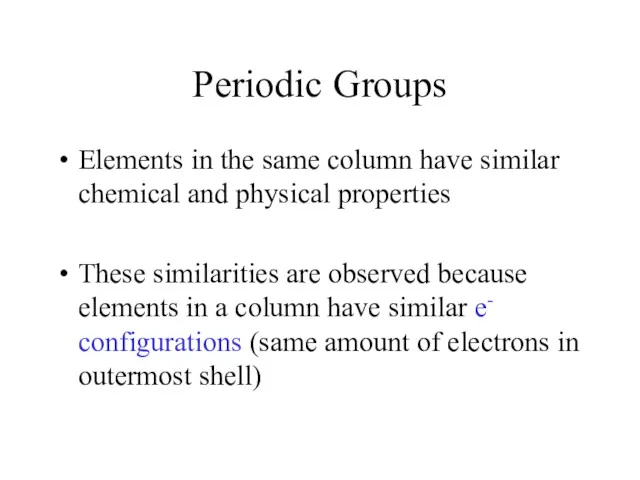
- 5. Periodic Groups Elements in the same column have similar chemical and physical properties These similarities are
- 6. Periodic Trends Periodic Trends – patterns (don’t always hold true) can be seen with our current
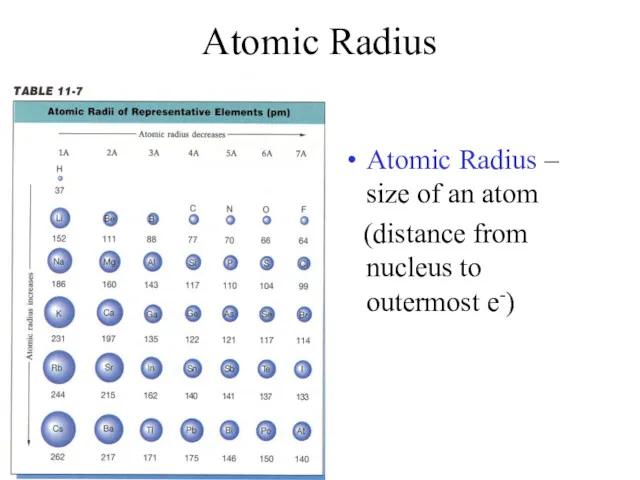
- 7. Atomic Radius Atomic Radius – size of an atom (distance from nucleus to outermost e-)
- 8. Atomic Radius Trend Group Trend – As you go down a column, atomic radius increases As
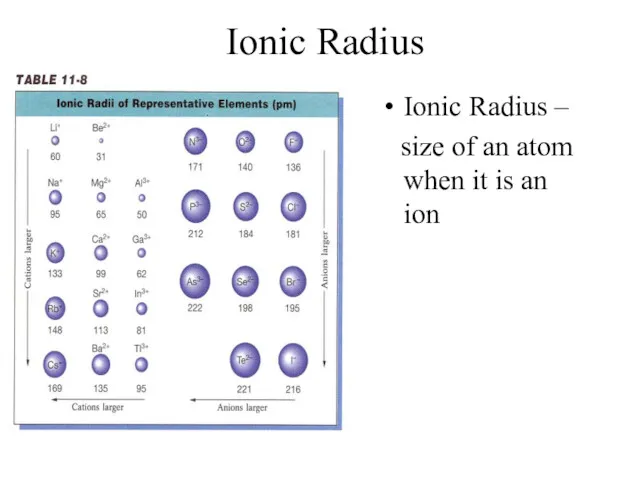
- 9. Ionic Radius Ionic Radius – size of an atom when it is an ion
- 10. Ionic Radius Trend Metals – lose e-, which means more p+ than e- (more attraction) SO…
- 11. Ionic Radius Trend Group Trend – As you go down a column, ionic radius increases Periodic
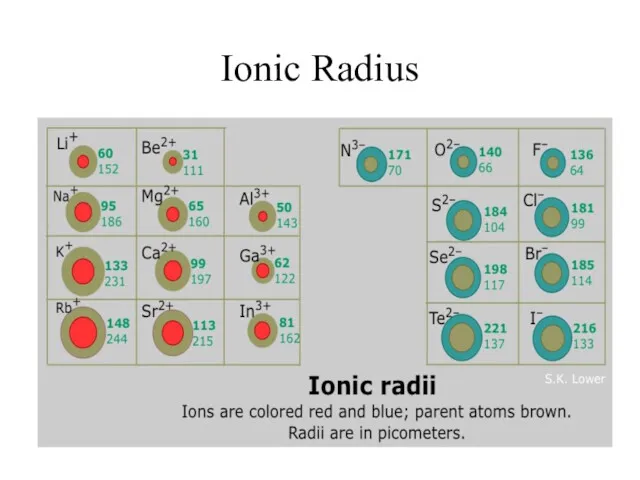
- 12. Ionic Radius
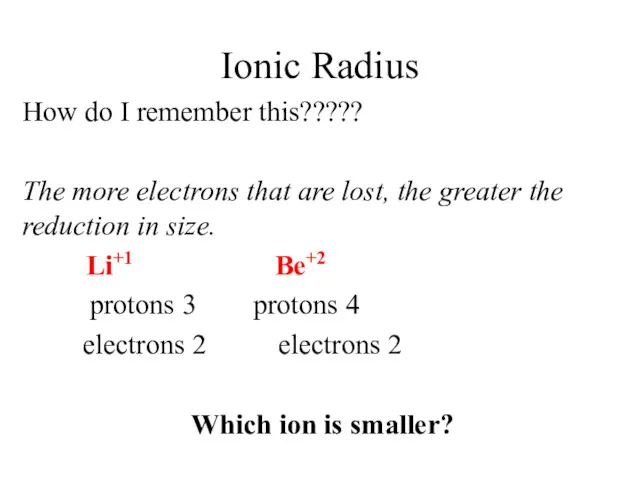
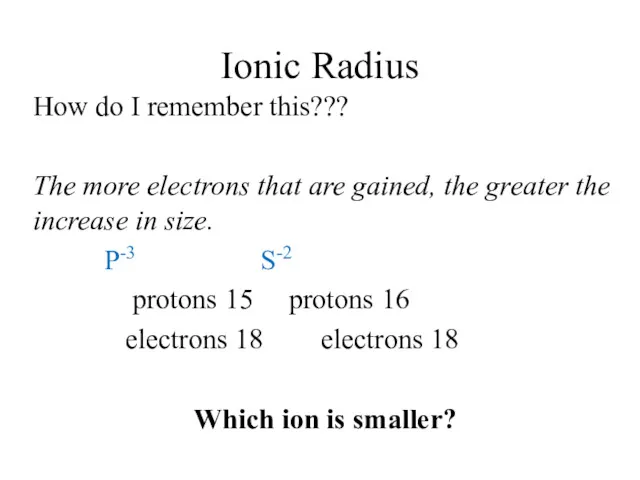
- 13. Ionic Radius How do I remember this????? The more electrons that are lost, the greater the
- 14. Ionic Radius How do I remember this??? The more electrons that are gained, the greater the
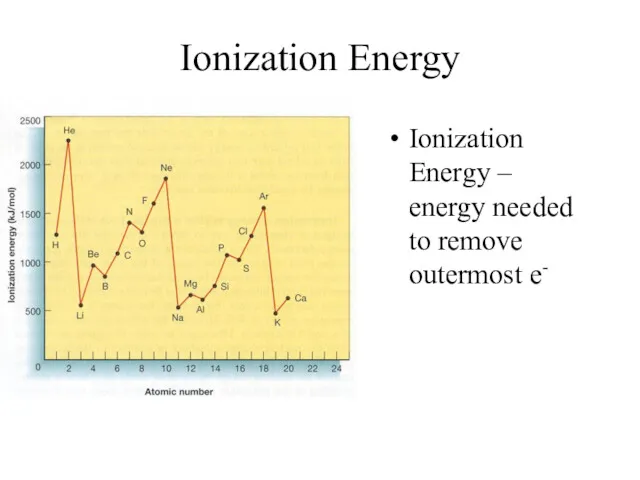
- 15. Ionization Energy Ionization Energy – energy needed to remove outermost e-
- 16. Ionization Energy Group Trend – As you go down a column, ionization energy decreases As you
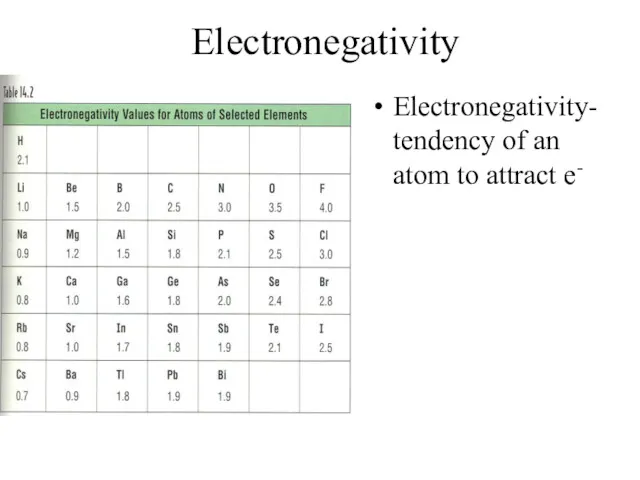
- 17. Electronegativity Electronegativity- tendency of an atom to attract e-
- 18. Electronegativity Trend Group Trend – As you go down a column, electronegativity decreases As you go
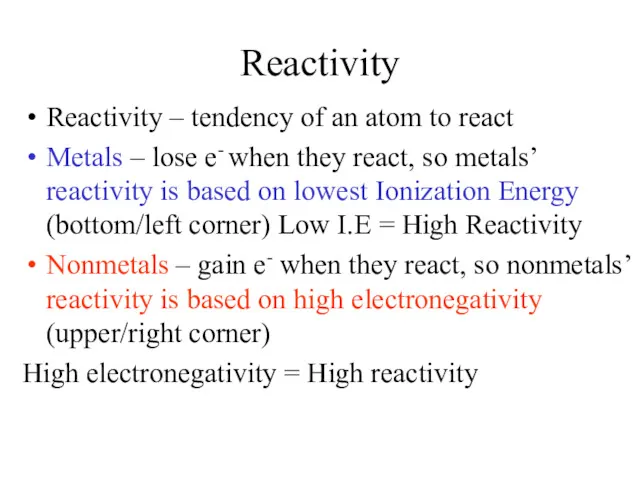
- 19. Reactivity Reactivity – tendency of an atom to react Metals – lose e- when they react,
- 21. Скачать презентацию






 Строение атома в соответствии с положением в периодической системе химических элементов Д.И. Менделеева
Строение атома в соответствии с положением в периодической системе химических элементов Д.И. Менделеева Алкадиены (диены, диеновые углеводороды)
Алкадиены (диены, диеновые углеводороды) Алканы в природе
Алканы в природе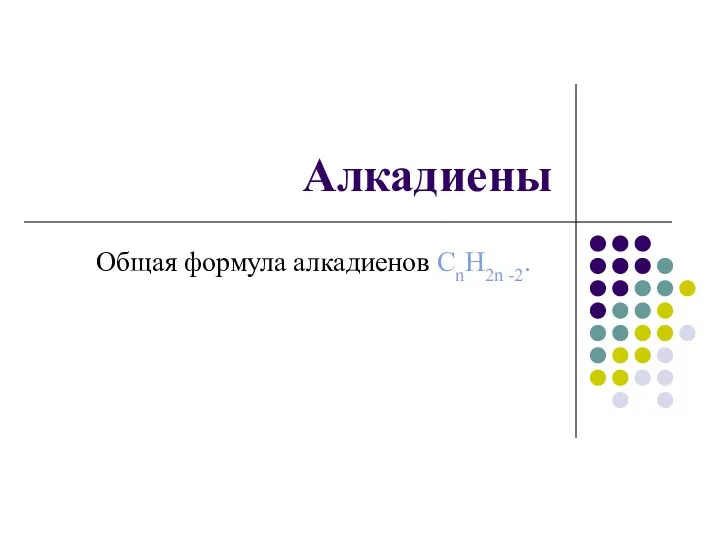 Алкадиены. Общая формула алкадиенов CnH2n -2
Алкадиены. Общая формула алкадиенов CnH2n -2 Обзор электродных процессов
Обзор электродных процессов Водород, получение, свойства и применение
Водород, получение, свойства и применение Предмет органической химии. Органические вещества
Предмет органической химии. Органические вещества Нуклеиновые кислоты
Нуклеиновые кислоты Получение пресной и чистой воды
Получение пресной и чистой воды Анализ проб воды
Анализ проб воды Виды изомерии в органической химии
Виды изомерии в органической химии Виды присадок к моторным топливам. Присадки к дизельному топливу
Виды присадок к моторным топливам. Присадки к дизельному топливу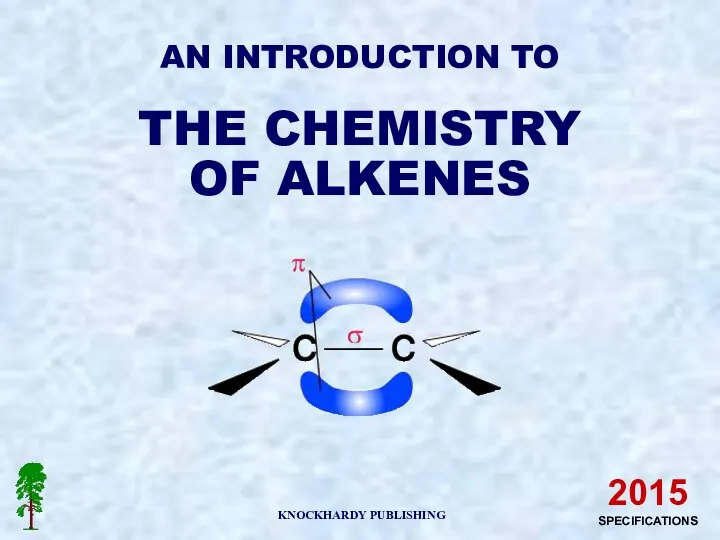 An introduction to the chemistry of alkenes
An introduction to the chemistry of alkenes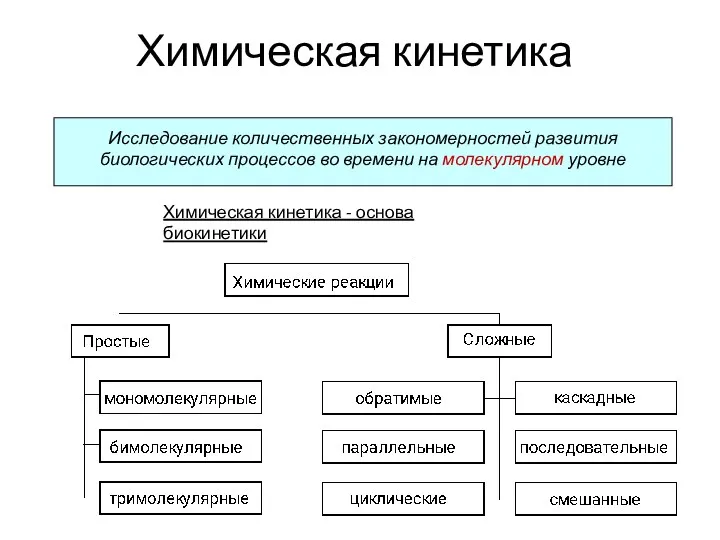 Химическая кинетика
Химическая кинетика Периодическая система химических элементов. Знаки химических элементов
Периодическая система химических элементов. Знаки химических элементов Застосування алканів
Застосування алканів Формы минералов и их агрегатов
Формы минералов и их агрегатов Основы коррозии и защиты металлов. Химическая коррозия
Основы коррозии и защиты металлов. Химическая коррозия Процессы и аппараты химических производств
Процессы и аппараты химических производств Тема 10- Гетроциклические соединения
Тема 10- Гетроциклические соединения Метод МО
Метод МО Металлы в нашей жизни
Металлы в нашей жизни Основные электрохимические процессы
Основные электрохимические процессы Химия в быту
Химия в быту Электрохимические процессы. Лекция 7
Электрохимические процессы. Лекция 7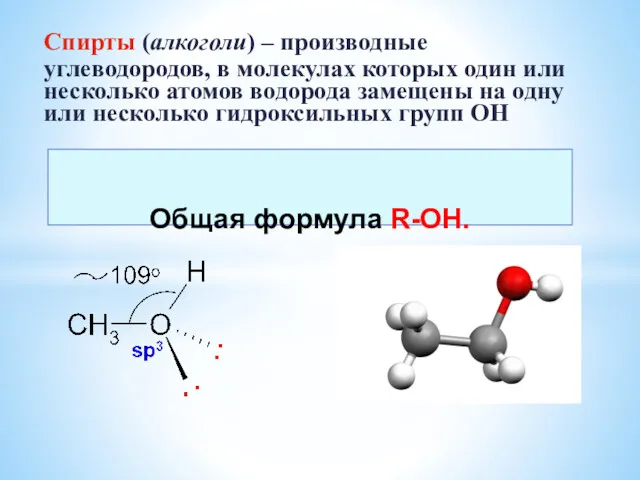 Спирты. Многоатомные спирты
Спирты. Многоатомные спирты Азот: кислородные соединения. Особенности химии фосфора и элементов его подгруппы
Азот: кислородные соединения. Особенности химии фосфора и элементов его подгруппы Алюминий және оның қосылыстары
Алюминий және оның қосылыстары