Содержание
- 2. INTRODUCTION This Powerpoint show is one of several produced to help students understand selected topics at
- 3. CONTENTS Structure of alkenes Nomenclature Isomerism Physical properties of alkenes Electrophilic addition reactions of alkenes Addition
- 4. Before you start it would be helpful to… Recall the definition of a covalent bond Understand
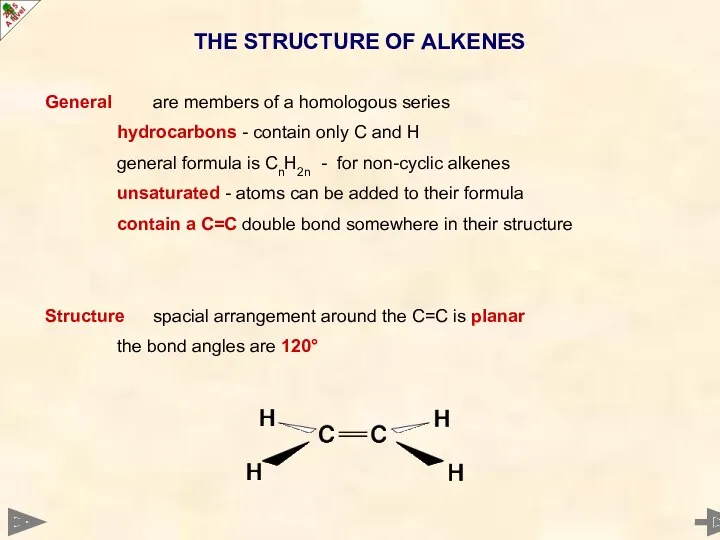
- 5. General are members of a homologous series hydrocarbons - contain only C and H general formula
- 6. General are members of a homologous series hydrocarbons - contain only C and H general formula
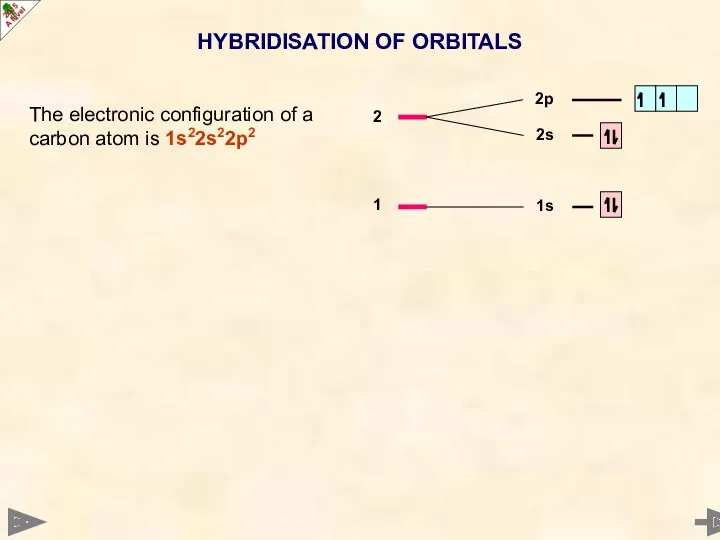
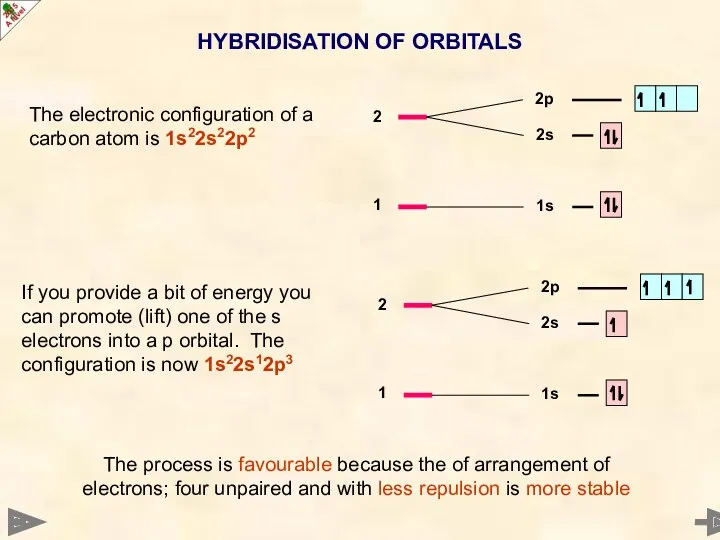
- 7. HYBRIDISATION OF ORBITALS The electronic configuration of a carbon atom is 1s22s22p2
- 8. HYBRIDISATION OF ORBITALS The electronic configuration of a carbon atom is 1s22s22p2 If you provide a
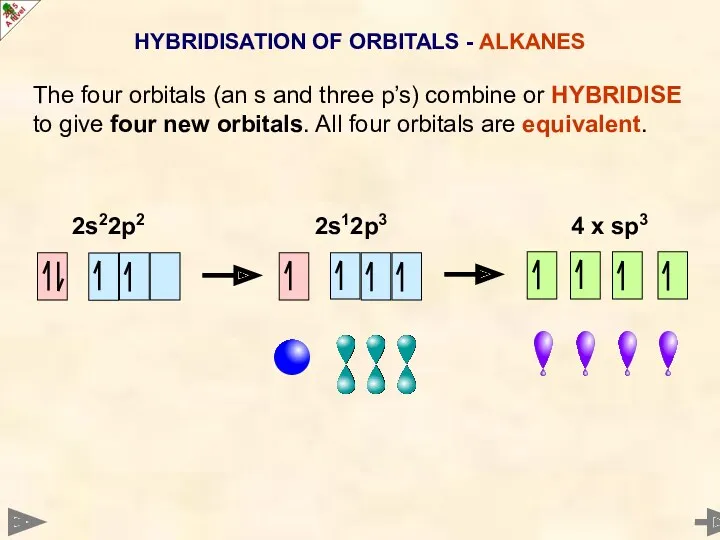
- 9. HYBRIDISATION OF ORBITALS - ALKANES The four orbitals (an s and three p’s) combine or HYBRIDISE
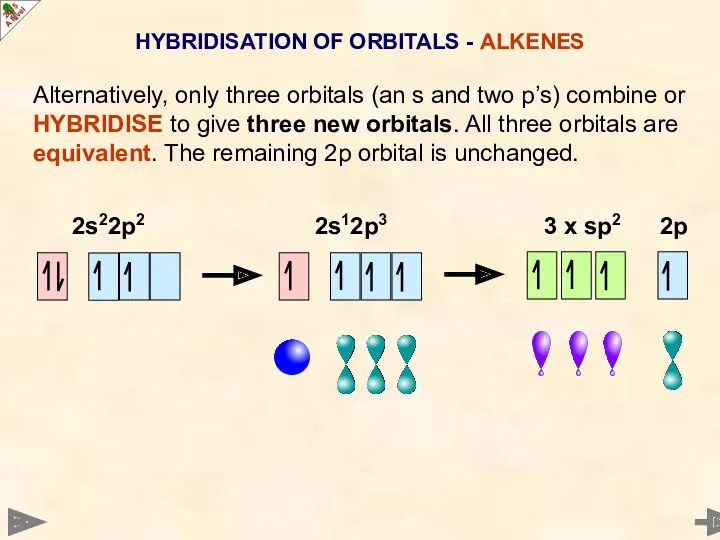
- 10. HYBRIDISATION OF ORBITALS - ALKENES Alternatively, only three orbitals (an s and two p’s) combine or
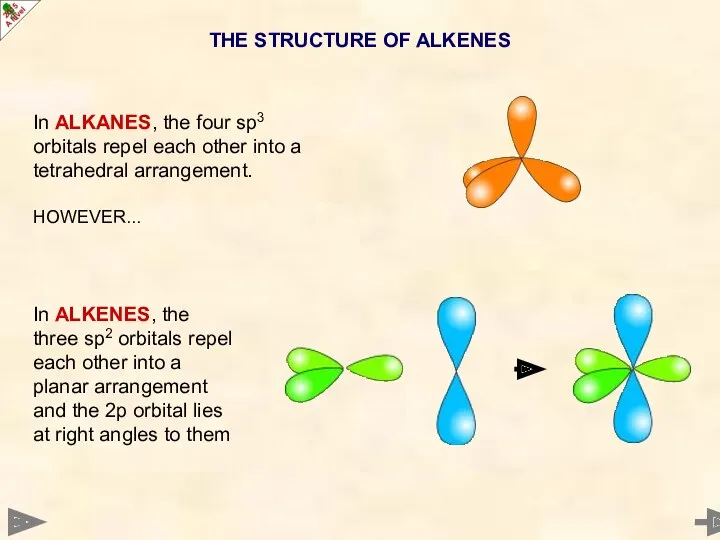
- 11. In ALKANES, the four sp3 orbitals repel each other into a tetrahedral arrangement. HOWEVER... In ALKENES,
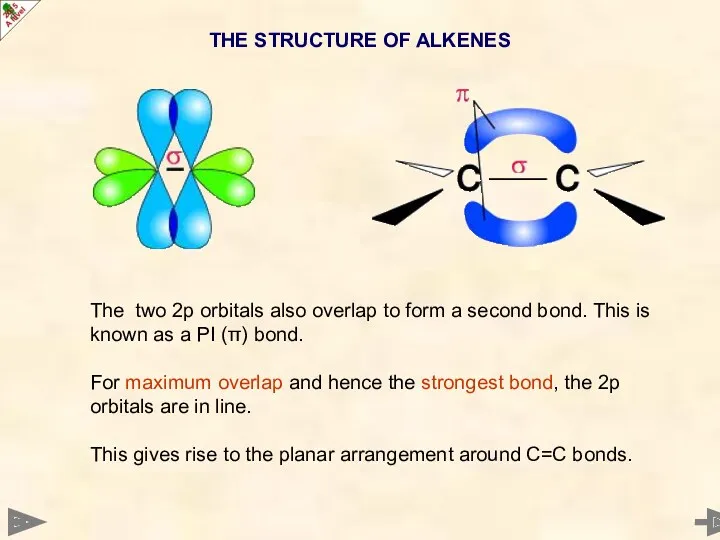
- 12. Covalent bonds are formed by overlap of orbitals. An sp2 orbital from each carbon overlaps to
- 13. The two 2p orbitals also overlap to form a second bond. This is known as a
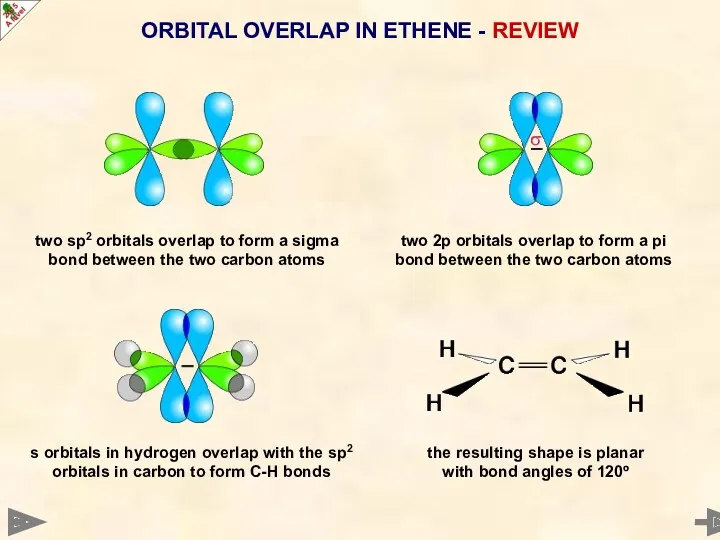
- 14. two sp2 orbitals overlap to form a sigma bond between the two carbon atoms ORBITAL OVERLAP
- 15. Alkenes are named according to standard IUPAC rules • select the longest chain of C atoms
- 16. ISOMERISM IN ALKENES Two types of isomerism found in alkenes STRUCTURAL GEOMETRICAL
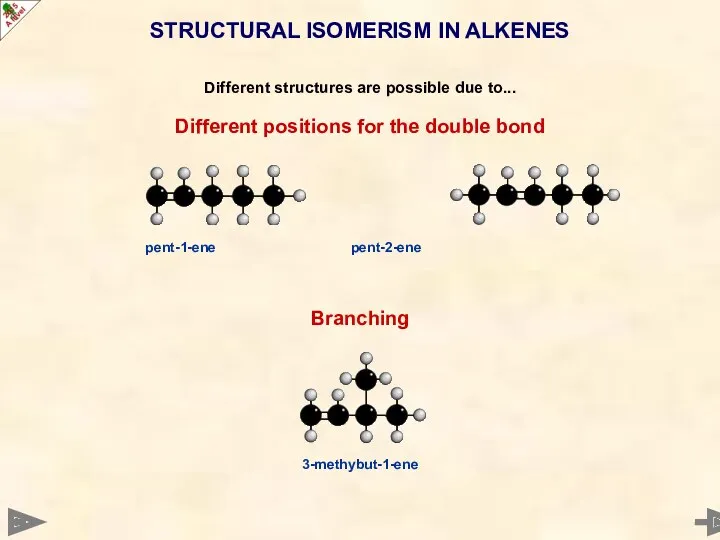
- 17. STRUCTURAL ISOMERISM IN ALKENES Different structures are possible due to... Different positions for the double bond
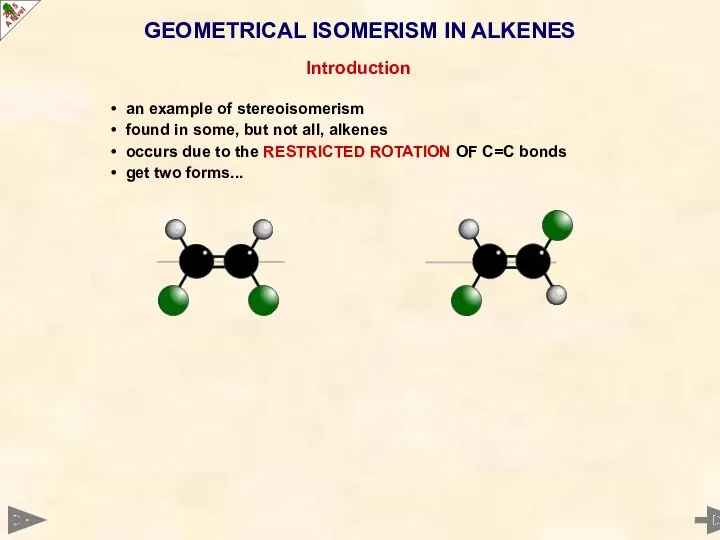
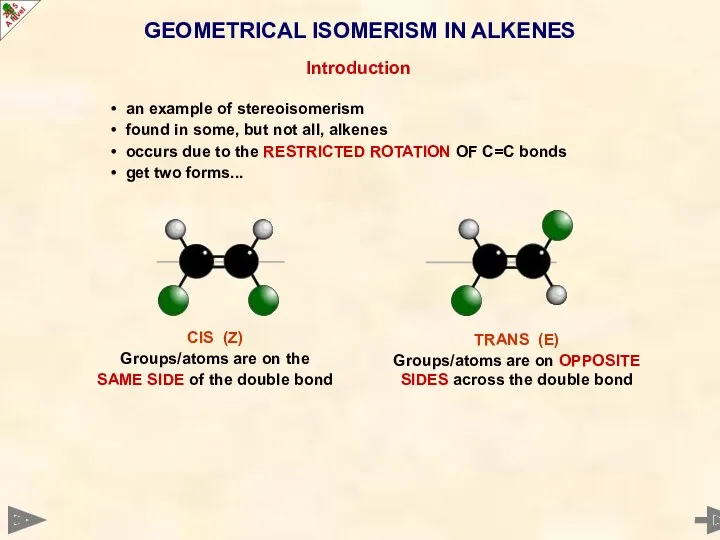
- 18. GEOMETRICAL ISOMERISM IN ALKENES Introduction an example of stereoisomerism found in some, but not all, alkenes
- 19. GEOMETRICAL ISOMERISM IN ALKENES Introduction an example of stereoisomerism found in some, but not all, alkenes
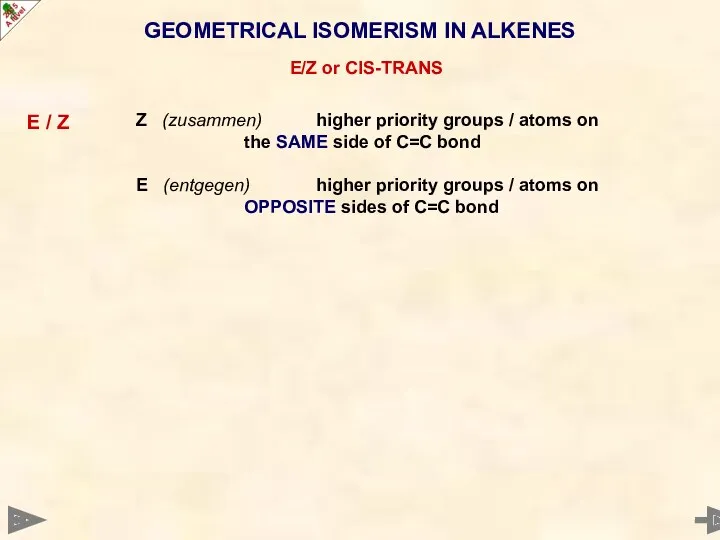
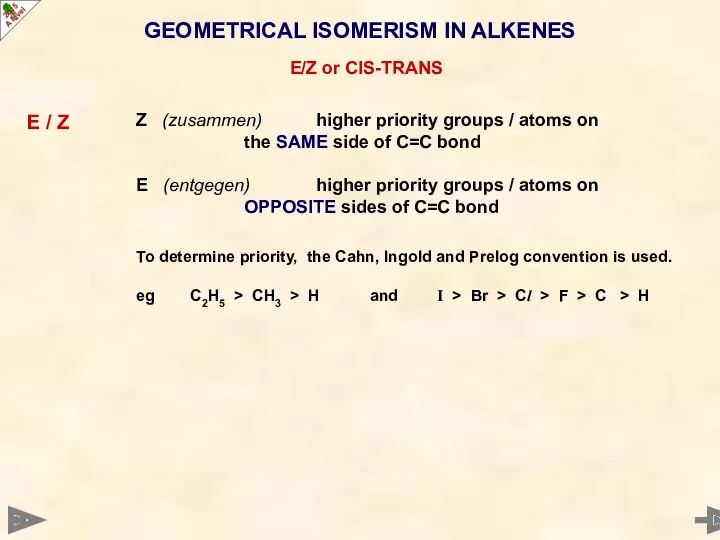
- 20. GEOMETRICAL ISOMERISM IN ALKENES E/Z or CIS-TRANS E / Z Z (zusammen) higher priority groups /
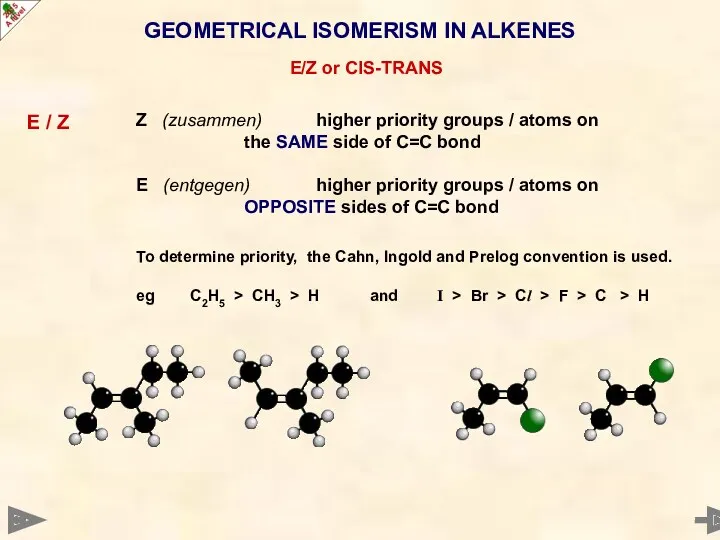
- 21. GEOMETRICAL ISOMERISM IN ALKENES E/Z or CIS-TRANS E / Z Z (zusammen) higher priority groups /
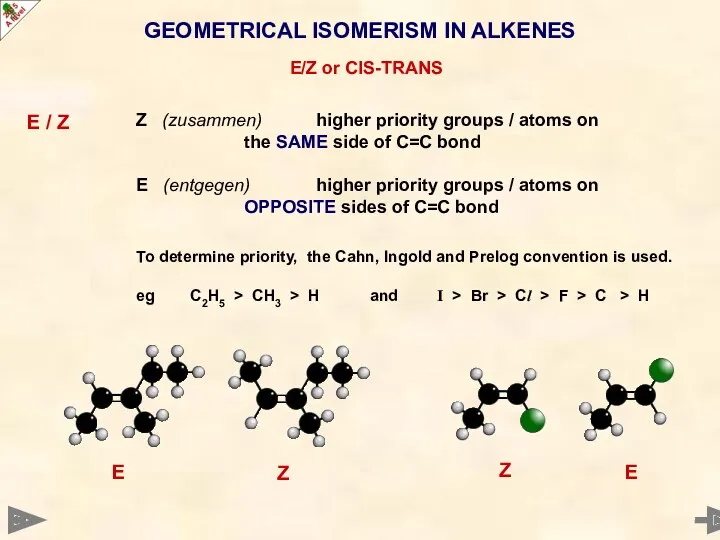
- 22. GEOMETRICAL ISOMERISM IN ALKENES E/Z or CIS-TRANS E / Z Z (zusammen) higher priority groups /
- 23. GEOMETRICAL ISOMERISM IN ALKENES E/Z or CIS-TRANS E / Z Z (zusammen) higher priority groups /
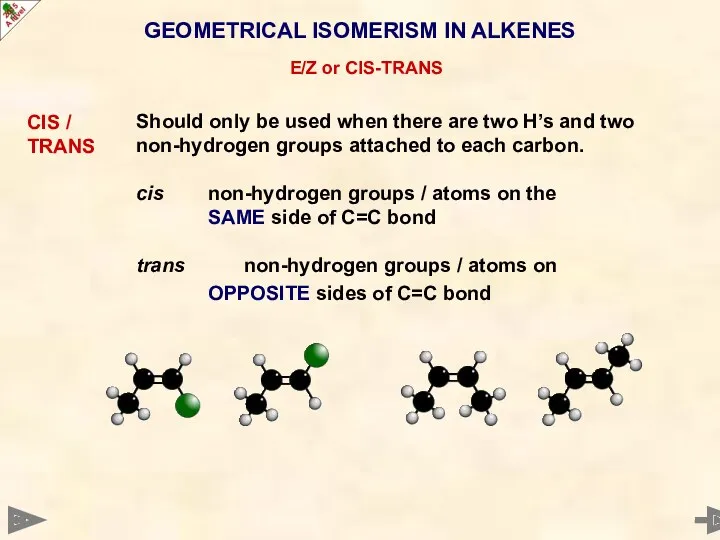
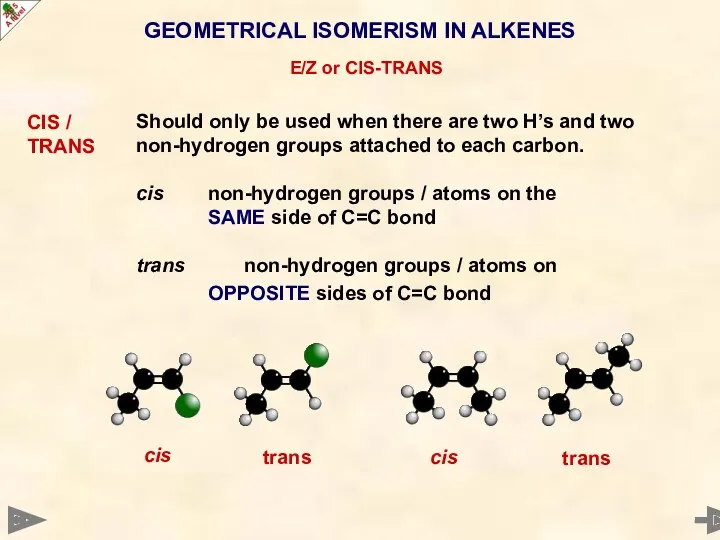
- 24. GEOMETRICAL ISOMERISM IN ALKENES E/Z or CIS-TRANS CIS / TRANS Should only be used when there
- 25. GEOMETRICAL ISOMERISM IN ALKENES E/Z or CIS-TRANS CIS / TRANS Should only be used when there
- 26. GEOMETRICAL ISOMERISM IN ALKENES E/Z or CIS-TRANS CIS / TRANS Should only be used when there
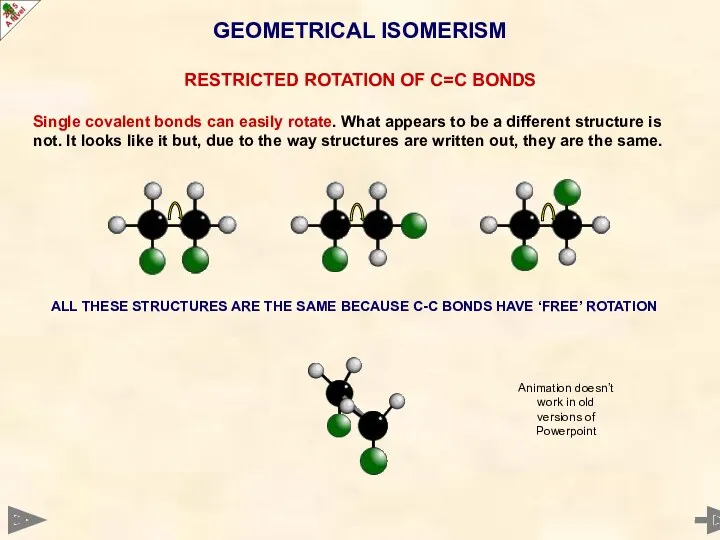
- 27. GEOMETRICAL ISOMERISM RESTRICTED ROTATION OF C=C BONDS Single covalent bonds can easily rotate. What appears to
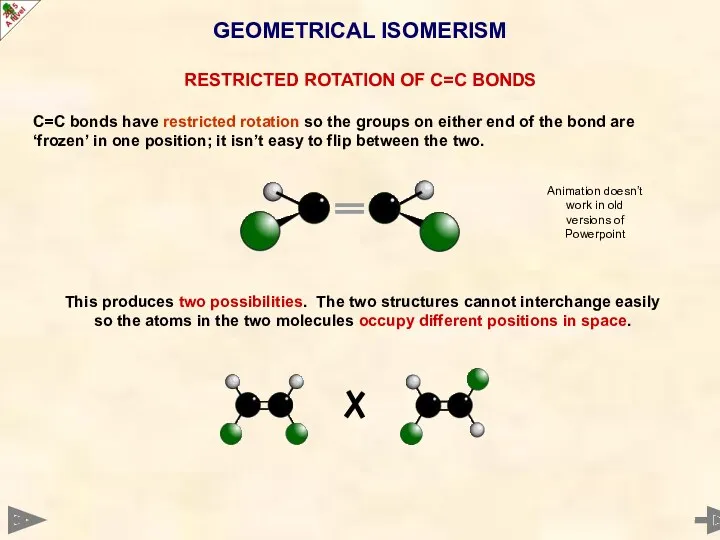
- 28. GEOMETRICAL ISOMERISM RESTRICTED ROTATION OF C=C BONDS C=C bonds have restricted rotation so the groups on
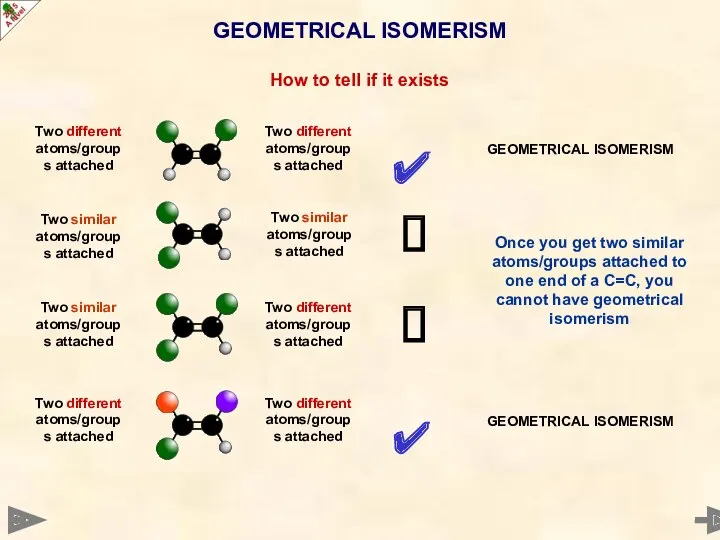
- 29. GEOMETRICAL ISOMERISM How to tell if it exists ✔ ? ? ✔ Two different atoms/groups attached
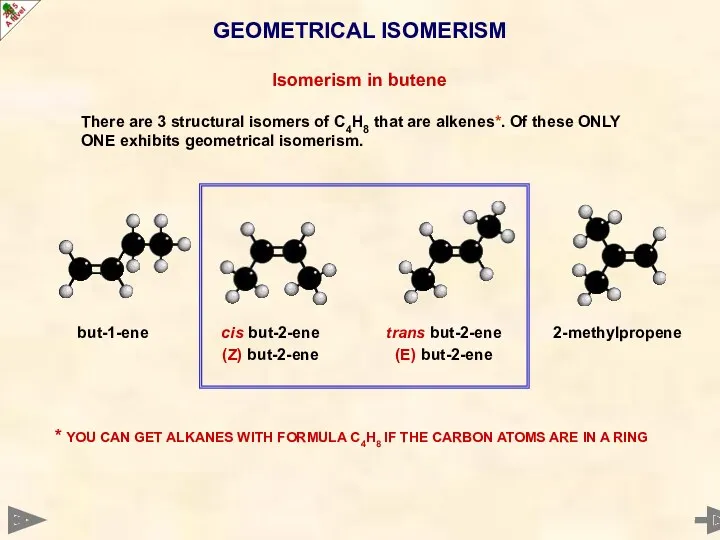
- 30. GEOMETRICAL ISOMERISM Isomerism in butene There are 3 structural isomers of C4H8 that are alkenes*. Of
- 31. Boiling point trends are similar to those shown in alkanes increases as they get more carbon
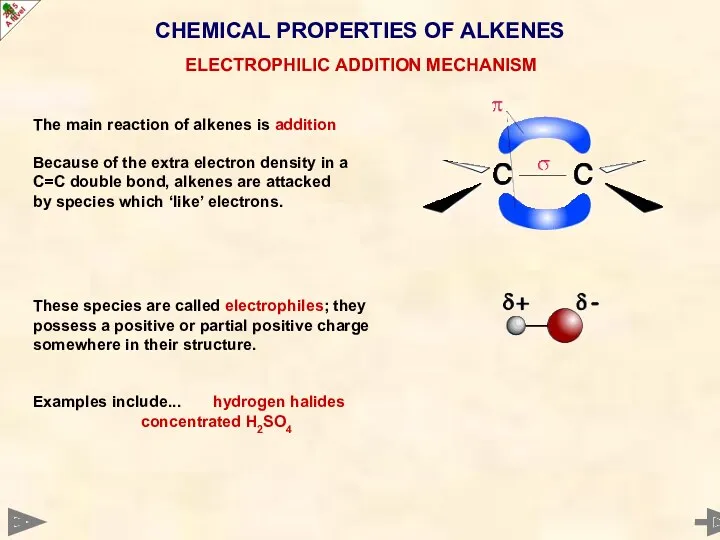
- 32. CHEMICAL PROPERTIES OF ALKENES ELECTROPHILIC ADDITION MECHANISM The main reaction of alkenes is addition Because of
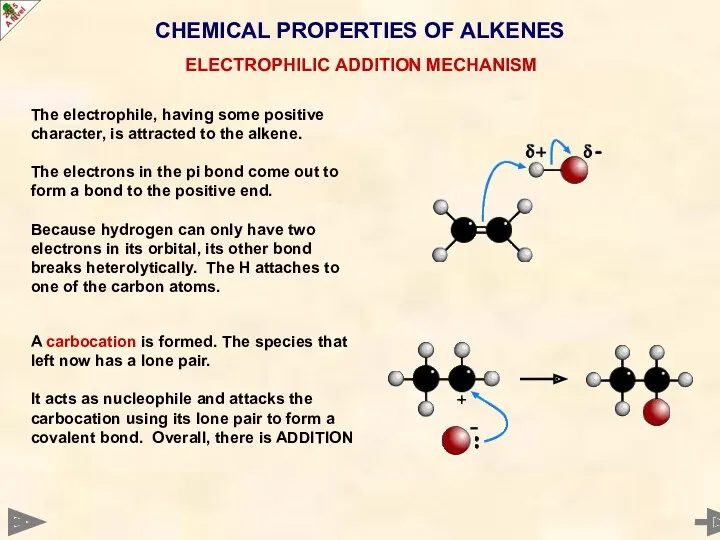
- 33. CHEMICAL PROPERTIES OF ALKENES ELECTROPHILIC ADDITION MECHANISM The electrophile, having some positive character, is attracted to
- 34. CHEMICAL PROPERTIES OF ALKENES ELECTROPHILIC ADDITION MECHANISM The electrophile, having some positive character, is attracted to
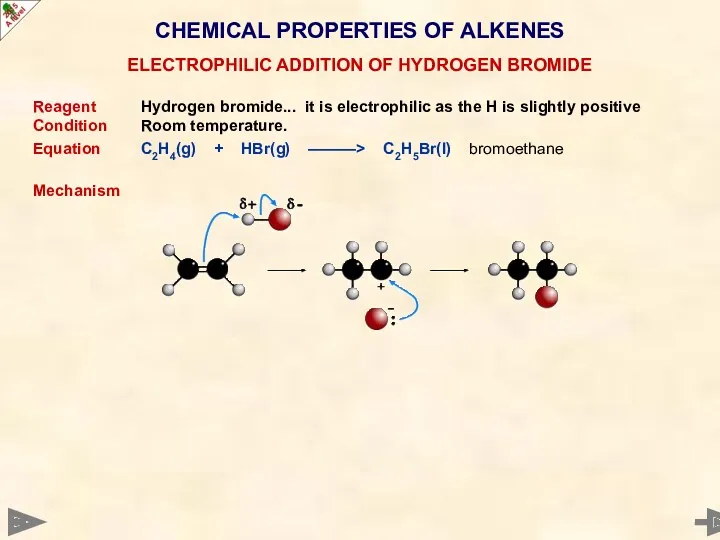
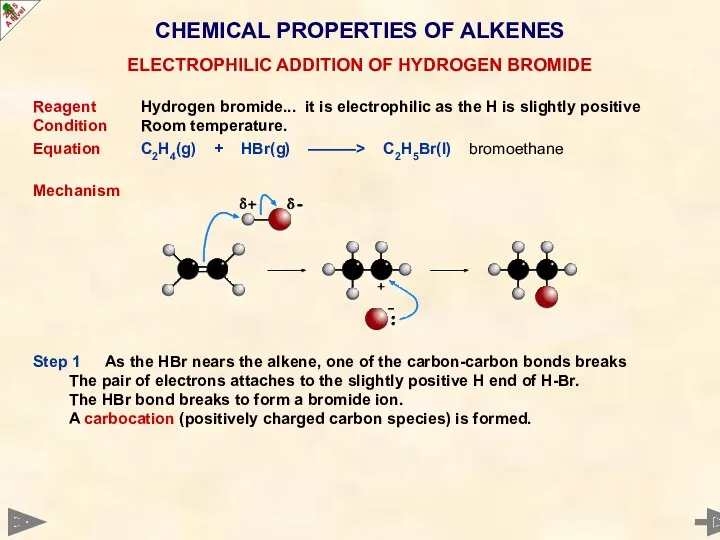
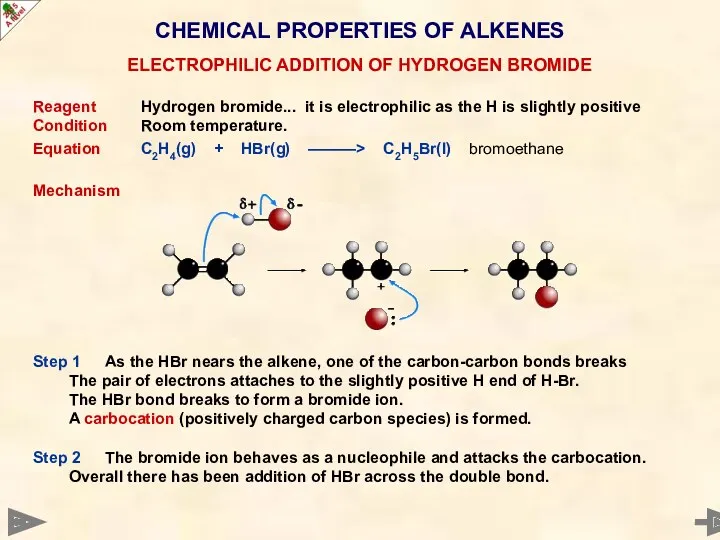
- 35. CHEMICAL PROPERTIES OF ALKENES Reagent Hydrogen bromide... it is electrophilic as the H is slightly positive
- 36. CHEMICAL PROPERTIES OF ALKENES Reagent Hydrogen bromide... it is electrophilic as the H is slightly positive
- 37. CHEMICAL PROPERTIES OF ALKENES Reagent Hydrogen bromide... it is electrophilic as the H is slightly positive
- 38. CHEMICAL PROPERTIES OF ALKENES ELECTROPHILIC ADDITION OF HYDROGEN BROMIDE ANIMATED MECHANISM Animation repeats continuously after every
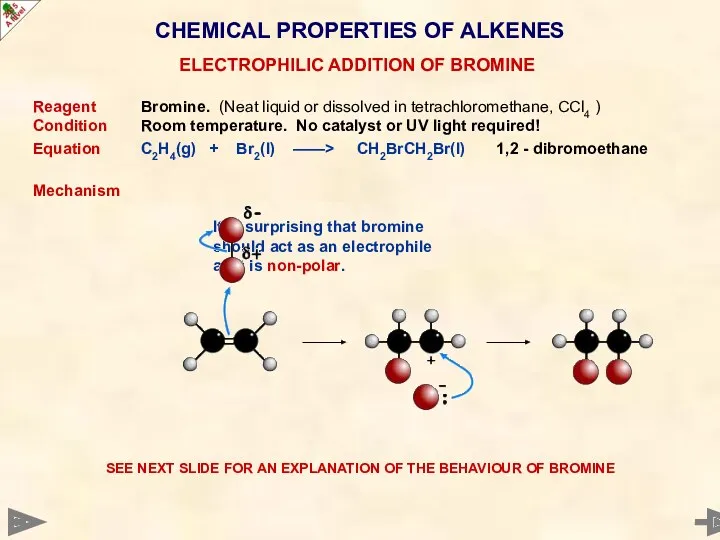
- 39. CHEMICAL PROPERTIES OF ALKENES Reagent Bromine. (Neat liquid or dissolved in tetrachloromethane, CCl4 ) Condition Room
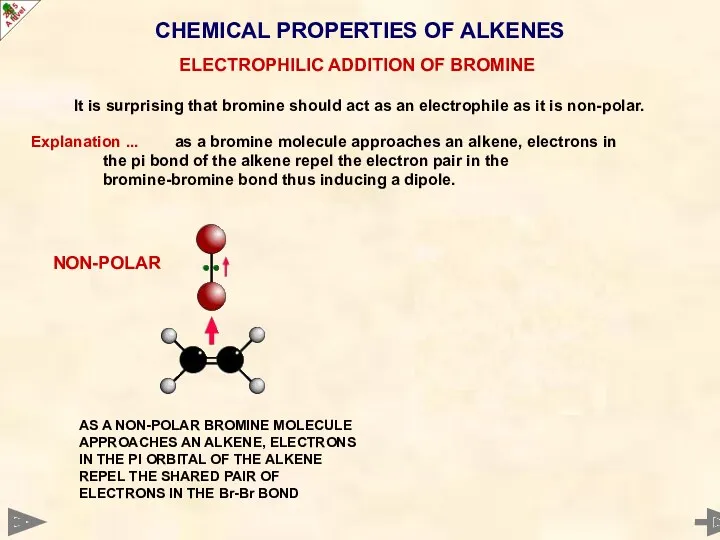
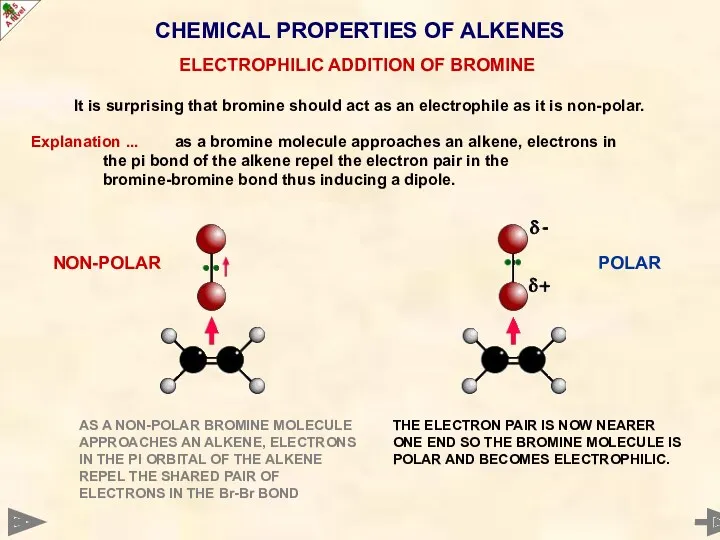
- 40. CHEMICAL PROPERTIES OF ALKENES It is surprising that bromine should act as an electrophile as it
- 41. CHEMICAL PROPERTIES OF ALKENES It is surprising that bromine should act as an electrophile as it
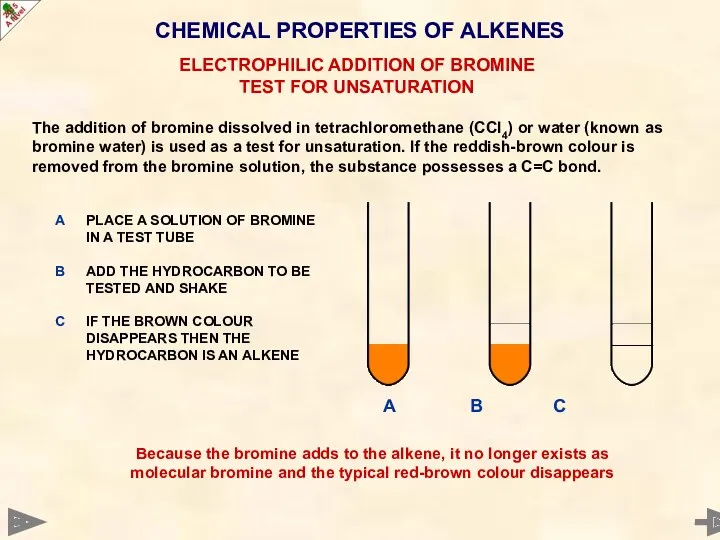
- 42. CHEMICAL PROPERTIES OF ALKENES The addition of bromine dissolved in tetrachloromethane (CCl4) or water (known as
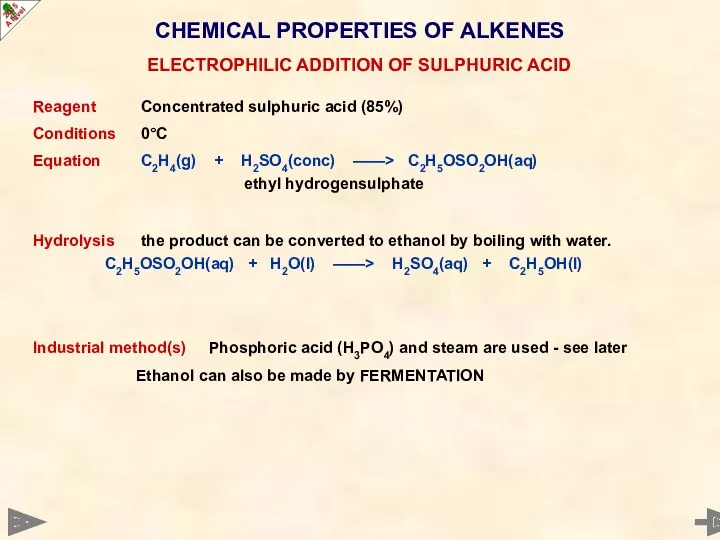
- 43. CHEMICAL PROPERTIES OF ALKENES Reagent Concentrated sulphuric acid (85%) Conditions 0°C Equation C2H4(g) + H2SO4(conc) ——>
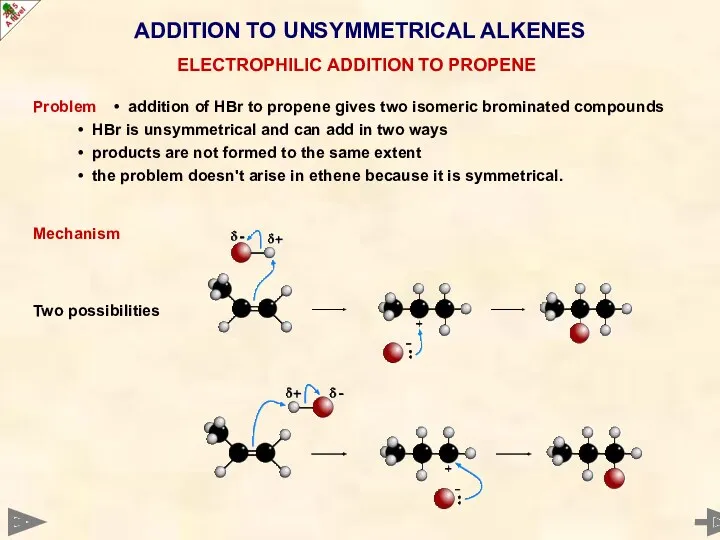
- 44. ADDITION TO UNSYMMETRICAL ALKENES Problem • addition of HBr to propene gives two isomeric brominated compounds
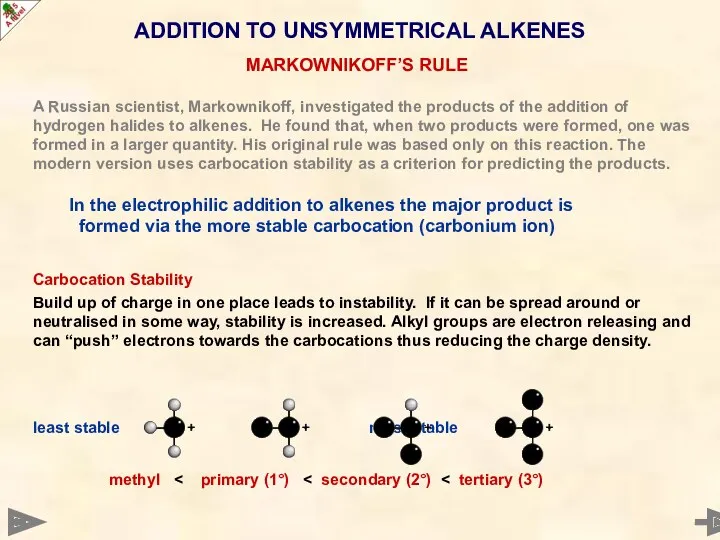
- 45. A Russian scientist, Markownikoff, investigated the products of the addition of hydrogen halides to alkenes. He
- 46. A Russian scientist, Markownikoff, investigated the products of the addition of hydrogen halides to alkenes. He
- 47. In the addition to propene, path A involves a 2° carbocation, path B a 1° carbocation.
- 48. ADDITION TO UNSYMMETRICAL ALKENES ELECTROPHILIC ADDITION TO PROPENE ANIMATED MECHANISM Animation repeats continuously after every 10
- 49. CHEMICAL PROPERTIES OF ALKENES DIRECT HYDRATION Reagent steam Conditions high pressure Catalyst phosphoric acid Product alcohol
- 50. CHEMICAL PROPERTIES OF ALKENES HYDROGENATION Reagent hydrogen Conditions nickel catalyst - finely divided Product alkanes Equation
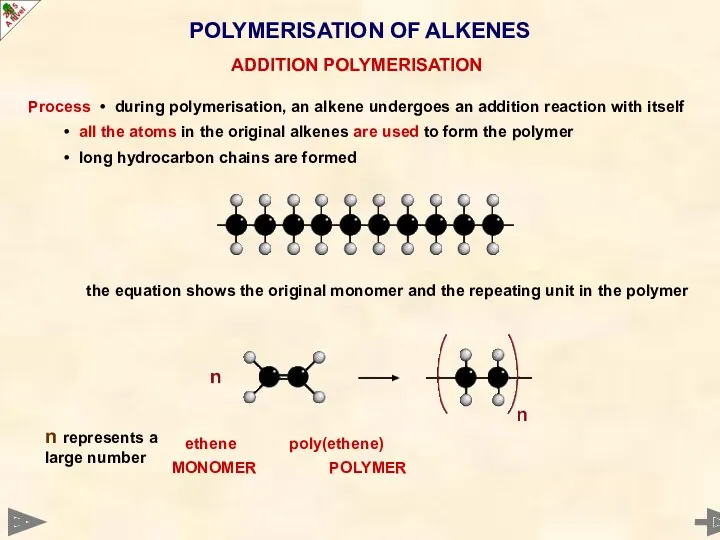
- 51. POLYMERISATION OF ALKENES Process • during polymerisation, an alkene undergoes an addition reaction with itself •
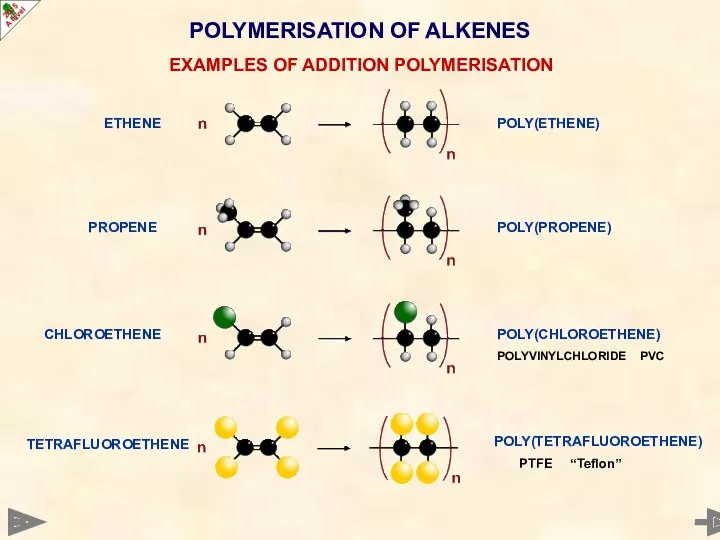
- 52. POLYMERISATION OF ALKENES ETHENE EXAMPLES OF ADDITION POLYMERISATION PROPENE TETRAFLUOROETHENE CHLOROETHENE POLY(ETHENE) POLY(PROPENE) POLY(CHLOROETHENE) POLYVINYLCHLORIDE PVC
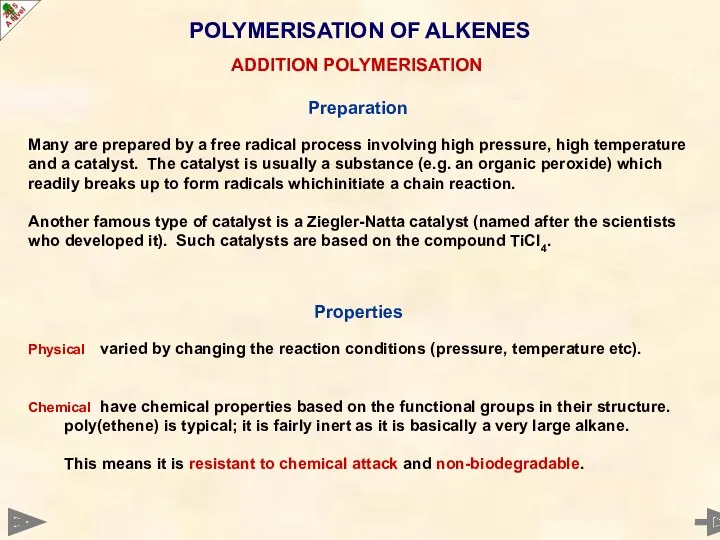
- 53. Preparation Many are prepared by a free radical process involving high pressure, high temperature and a
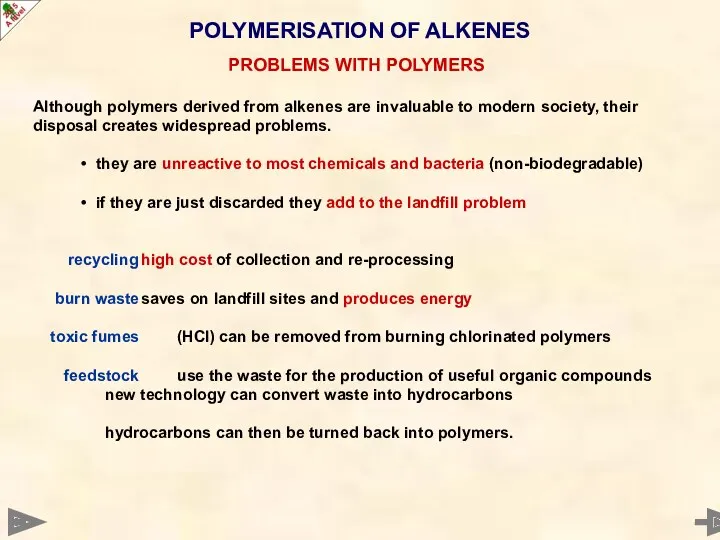
- 54. POLYMERISATION OF ALKENES Although polymers derived from alkenes are invaluable to modern society, their disposal creates
- 55. PREPARATION OF ALKENES FROM HALOGENOALKANES - Elimination Reagent Alcoholic sodium (or potassium) hydroxide Conditions Reflux in
- 56. REVISION CHECK What should you be able to do? Recall and explain the physical properties of
- 57. You need to go over the relevant topic(s) again Click on the button to return to
- 58. WELL DONE! Try some past paper questions
- 60. Скачать презентацию


















 Галогены. Фтор, хлор, бром, йод, астат
Галогены. Фтор, хлор, бром, йод, астат Пропилен-алкены(пропен)
Пропилен-алкены(пропен) Теория строения органических соединений
Теория строения органических соединений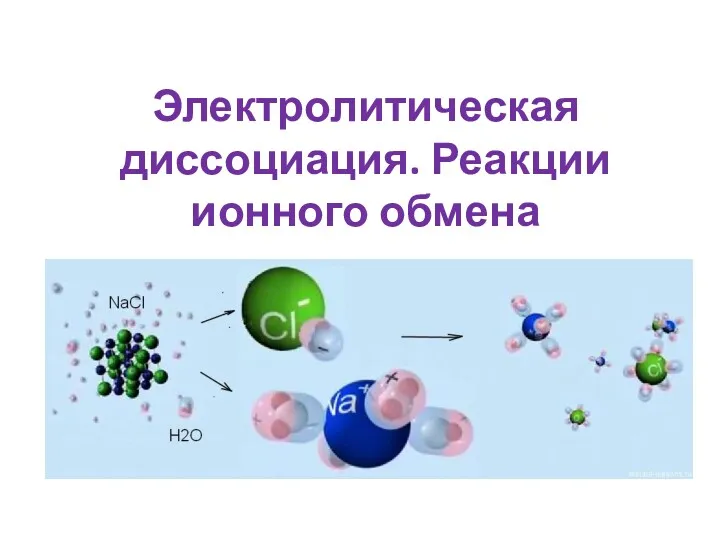 Электролитическая диссоциация. Реакции ионного обмена
Электролитическая диссоциация. Реакции ионного обмена 20230205_okislitelno-_vosstanovitelnye_reaktsii
20230205_okislitelno-_vosstanovitelnye_reaktsii Скорость химических реакций. Катализ. Химическое равновесие
Скорость химических реакций. Катализ. Химическое равновесие Соли. Классификация. Физические и химические свойства солей
Соли. Классификация. Физические и химические свойства солей Current Condition(Revision C)
Current Condition(Revision C) Состояние электронов в атоме
Состояние электронов в атоме Измерение и оценка химического фактора. Лекция 9
Измерение и оценка химического фактора. Лекция 9 Фосфор и его соединения
Фосфор и его соединения Прочность полимеров
Прочность полимеров Скорость химических реакций. Химическое равновесие
Скорость химических реакций. Химическое равновесие Бензол молекуласында байланыстардың түзілу сызбанұсқасы
Бензол молекуласында байланыстардың түзілу сызбанұсқасы Полімери. Їх властивості та застосування
Полімери. Їх властивості та застосування Периодический закон Д.И. Менделеева
Периодический закон Д.И. Менделеева Щелочные металлы
Щелочные металлы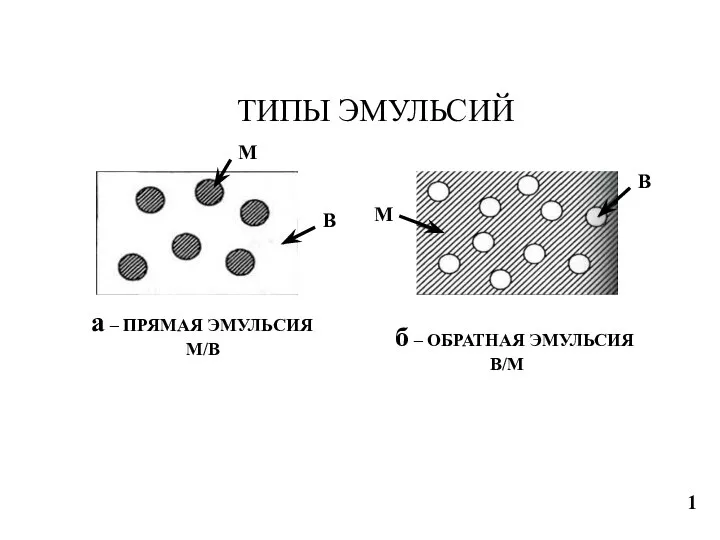 Типы эмульсий. Фракционный состав нефти. Типы нефти
Типы эмульсий. Фракционный состав нефти. Типы нефти Счастливый случай. Право первого хода
Счастливый случай. Право первого хода Газообразные вещества
Газообразные вещества Химическая коррозия. Методы защиты от химической коррозии
Химическая коррозия. Методы защиты от химической коррозии Алкины. Гомологический ряд
Алкины. Гомологический ряд Теория строения органического вещества. 10 класс
Теория строения органического вещества. 10 класс Самое удивительное на свете вещество - вода
Самое удивительное на свете вещество - вода АЛКАНЫ Строение молекулы метана.
АЛКАНЫ Строение молекулы метана. Сапалық талдау. Сапалық аналитикалық реакциялар
Сапалық талдау. Сапалық аналитикалық реакциялар Кислород. Химия. 8 класс
Кислород. Химия. 8 класс Физические свойства металлов
Физические свойства металлов