Содержание
- 2. Combustion, explosions, rusting, rotting, breathing
- 3. Oxidation numbers The oxidation state or oxidation number, is an indicator of the degree of oxidation
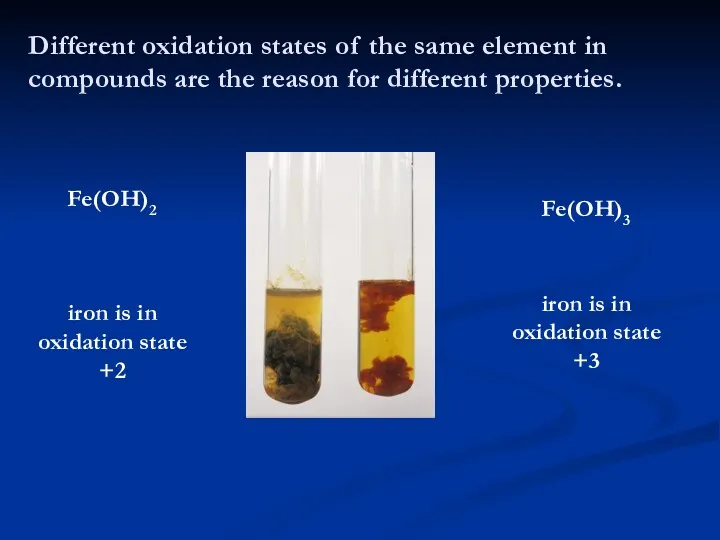
- 4. Different oxidation states of the same element in compounds are the reason for different properties. Fe(OH)2
- 5. Plutonium oxidation states
- 6. Determining the oxidation state or number Any pure element—even if it forms diatomic molecules like chlorine
- 7. Rules based on electronegativity Fluorine in compounds has an oxidation state of −1. Halogens other than
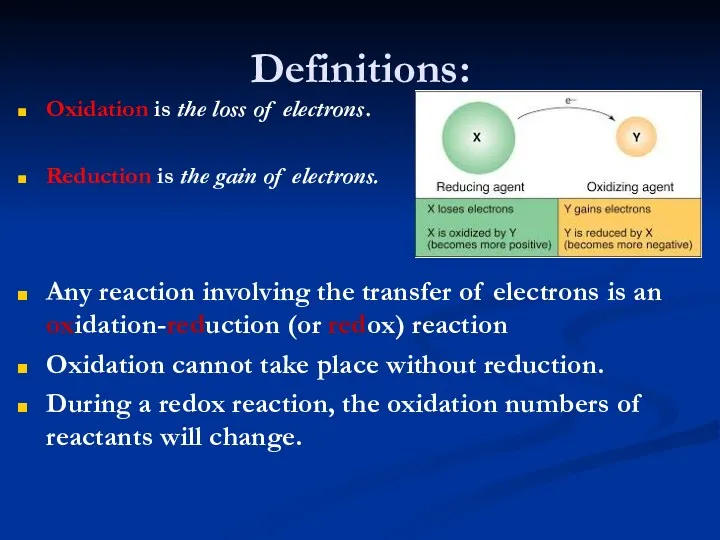
- 8. Definitions: Oxidation is the loss of electrons. Reduction is the gain of electrons. Any reaction involving
- 9. Oxidizing agents Substances that have the ability to oxidize other substances (cause them to lose electrons)
- 10. In the Thermit reaction, shown here, which substance is reduced and which is oxidized? Aluminium (Al)
- 11. Reducing agents Substances that have the ability to reduce other substances (cause them to gain electrons)
- 12. For any equation to be balanced: 1. The number of atoms of each type on the
- 13. Types of redox reactions 1. Combination General equation: A+B→AB 2 H2 + O2 = 2 H2O
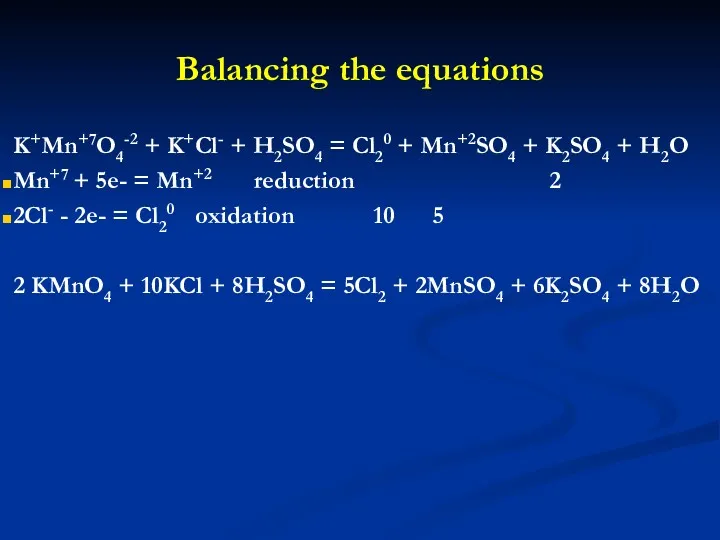
- 14. Balancing the equations K+Mn+7O4-2 + K+Cl- + H2SO4 = Cl20 + Mn+2SO4 + K2SO4 + H2O
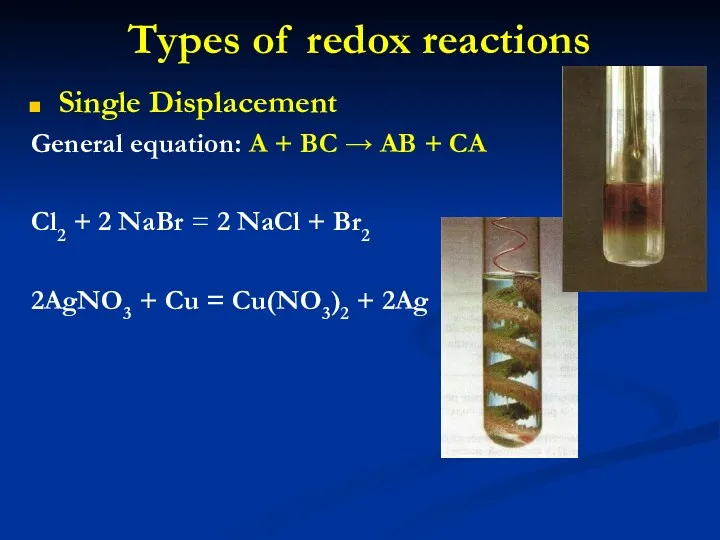
- 15. Types of redox reactions Single Displacement General equation: A + BC → AB + CA Cl2
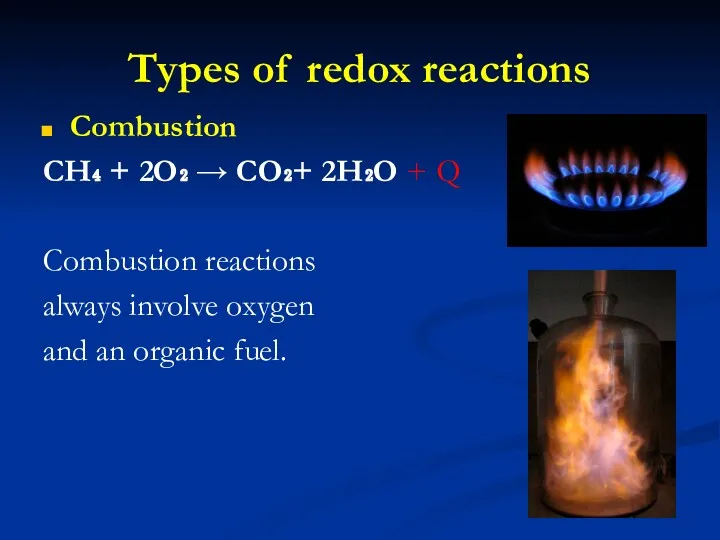
- 16. Types of redox reactions Combustion CH₄ + 2O₂ → CO₂+ 2H₂O + Q Combustion reactions always
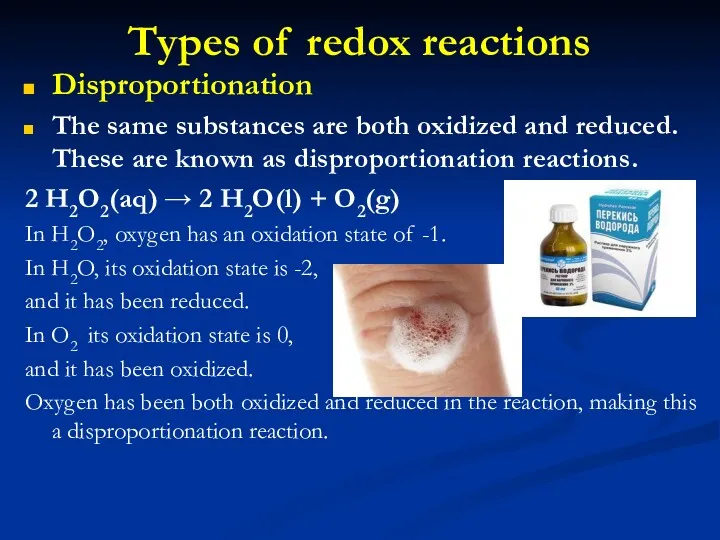
- 17. Types of redox reactions Disproportionation The same substances are both oxidized and reduced. These are known
- 19. Скачать презентацию








 Классификация химических реакций
Классификация химических реакций Как трудно быть особенной…
Как трудно быть особенной… Классификация кристаллов по типу химической связи
Классификация кристаллов по типу химической связи Химические свойства простых металлов, неметаллов и оксидов. Задание 6 по ЕГЭ
Химические свойства простых металлов, неметаллов и оксидов. Задание 6 по ЕГЭ Водород
Водород Физико-химия дисперсных систем. Коллоидные растворы
Физико-химия дисперсных систем. Коллоидные растворы Кремний и его соединения. К уроку химии в 9 классе
Кремний и его соединения. К уроку химии в 9 классе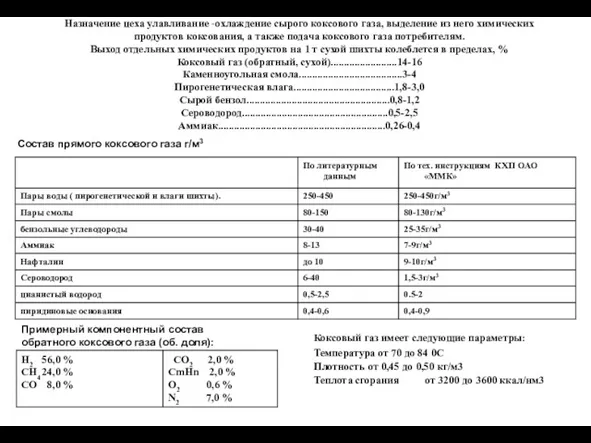 Охлаждение сырого коксового газа, выделение из него химических продуктов коксования
Охлаждение сырого коксового газа, выделение из него химических продуктов коксования Химические формулы. 8 класс
Химические формулы. 8 класс Химия в строительстве
Химия в строительстве Химия в повседневной жизни человека
Химия в повседневной жизни человека Алюминий и его соединения
Алюминий и его соединения Белоктар. Биохимиясы
Белоктар. Биохимиясы Свойства металлов
Свойства металлов Кислородсодержащие соединения серы SO2
Кислородсодержащие соединения серы SO2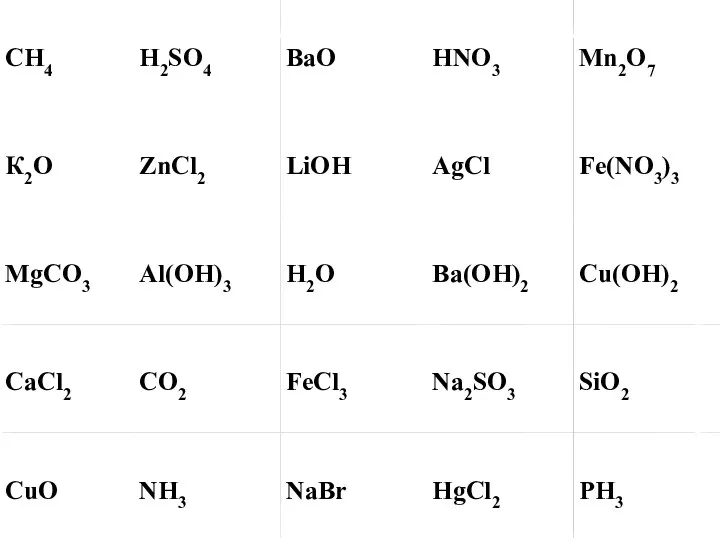 Химические реакции
Химические реакции Нефелометрический анализ
Нефелометрический анализ Удобрения
Удобрения Хімічні властивості оксидів
Хімічні властивості оксидів Тепловой эффект химических реакций
Тепловой эффект химических реакций Сущность хроматографии. Лекция 2-3
Сущность хроматографии. Лекция 2-3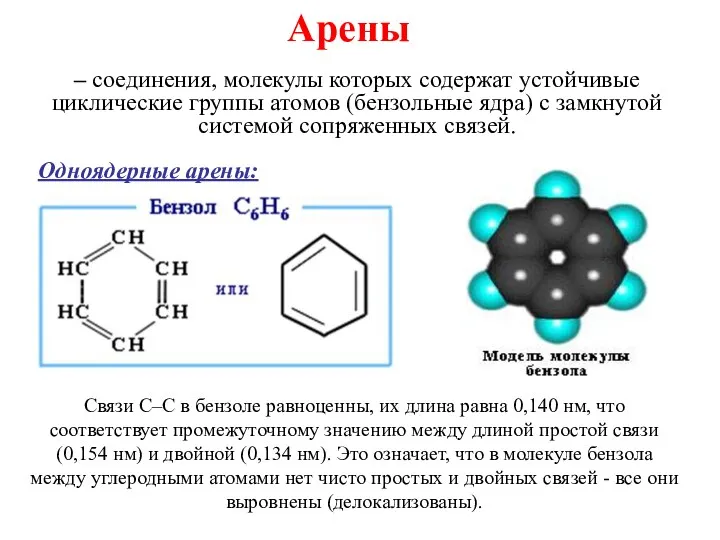 Ароматические углеводороды
Ароматические углеводороды Непредельные углеводороды. Алкены
Непредельные углеводороды. Алкены Оксиды
Оксиды Кобальт. Нахождение в природе. Получение
Кобальт. Нахождение в природе. Получение Electrochemistry. Oxidation-reduction equilibrium in water solutions
Electrochemistry. Oxidation-reduction equilibrium in water solutions Загрязнение продуктов питания
Загрязнение продуктов питания Методы окислительно-восстановительного титрования (Редоксиметрия)
Методы окислительно-восстановительного титрования (Редоксиметрия)