Содержание
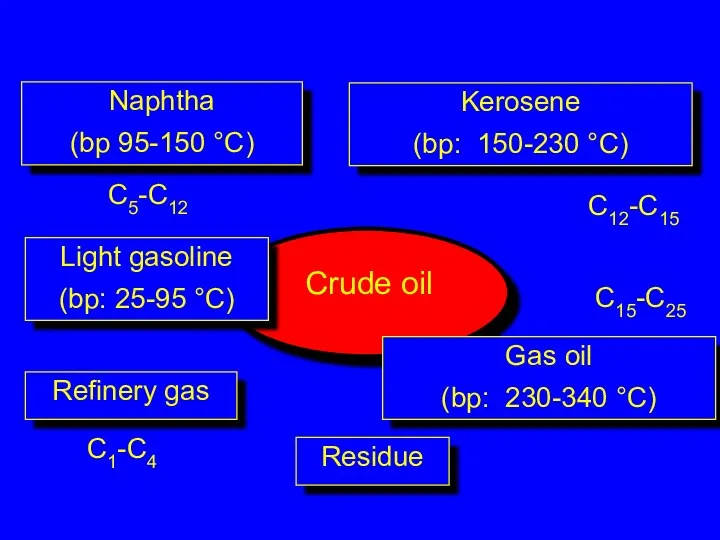
- 3. Refinery gas C1-C4 Light gasoline (bp: 25-95 °C) C5-C12 Naphtha (bp 95-150 °C) Kerosene (bp: 150-230
- 4. Petroleum refining Cracking converts high molecular weight hydrocarbons to more useful, low molecular weight ones Reforming
- 5. 2.14 Physical Properties of Alkanes and Cycloalkanes
- 6. Boiling Points of Alkanes governed by strength of intermolecular attractive forces alkanes are nonpolar, so dipole-dipole
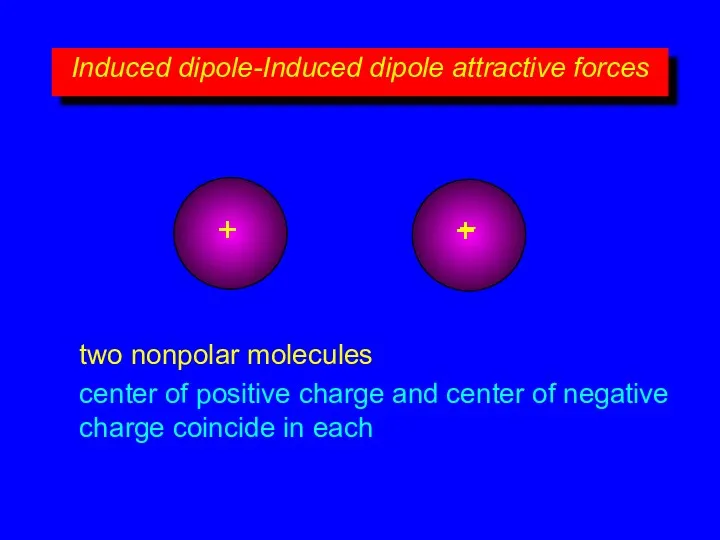
- 7. Induced dipole-Induced dipole attractive forces + – + – two nonpolar molecules center of positive charge
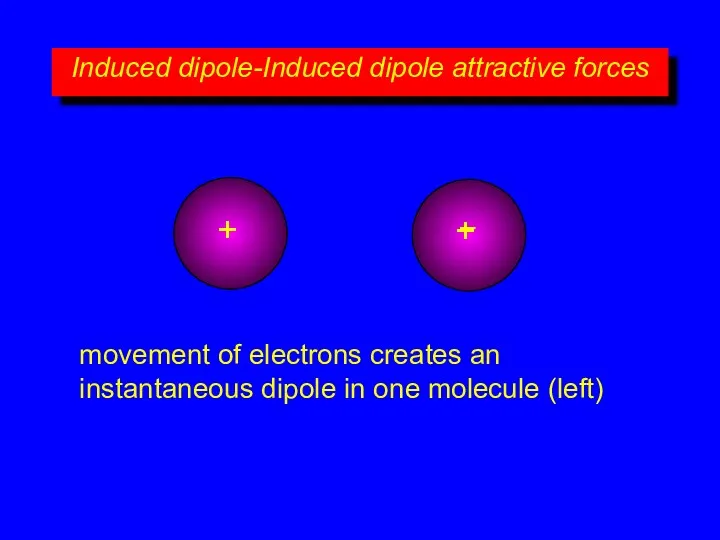
- 8. Induced dipole-Induced dipole attractive forces + – + – movement of electrons creates an instantaneous dipole
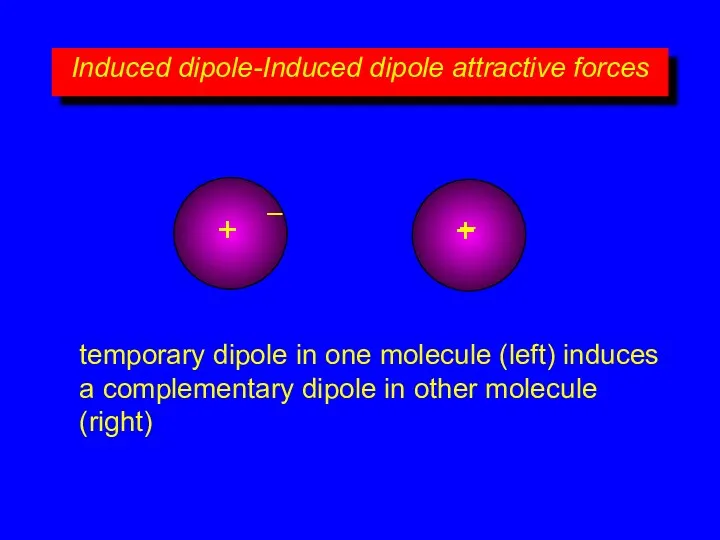
- 9. Induced dipole-Induced dipole attractive forces + – + – temporary dipole in one molecule (left) induces
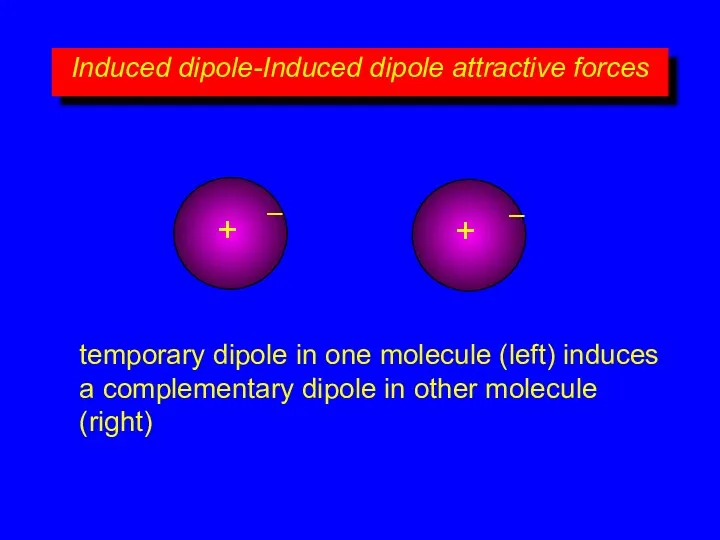
- 10. Induced dipole-Induced dipole attractive forces + – + – temporary dipole in one molecule (left) induces
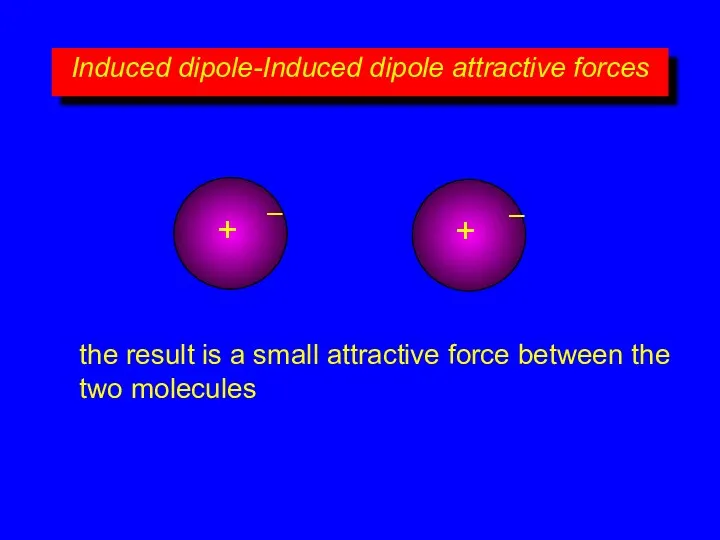
- 11. Induced dipole-Induced dipole attractive forces + – + – the result is a small attractive force
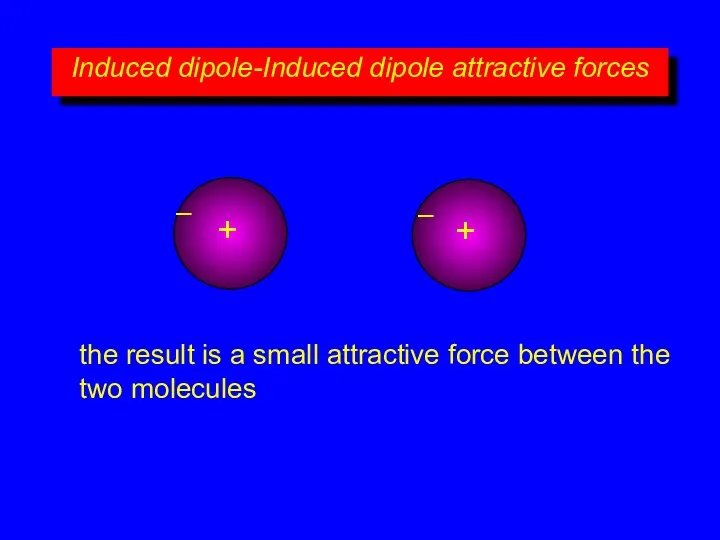
- 12. Induced dipole-Induced dipole attractive forces + – + – the result is a small attractive force
- 13. increase with increasing number of carbons more atoms, more electrons, more opportunities for induced dipole-induced dipole
- 14. increase with increasing number of carbons more atoms, more electrons, more opportunities for induced dipole-induced dipole
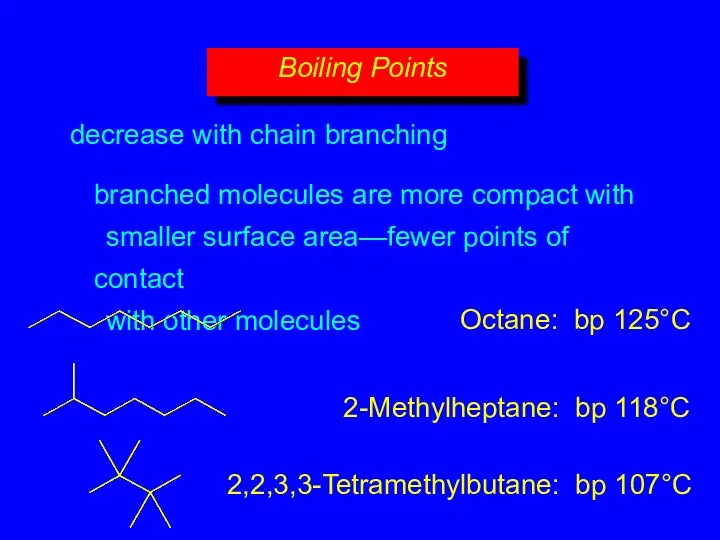
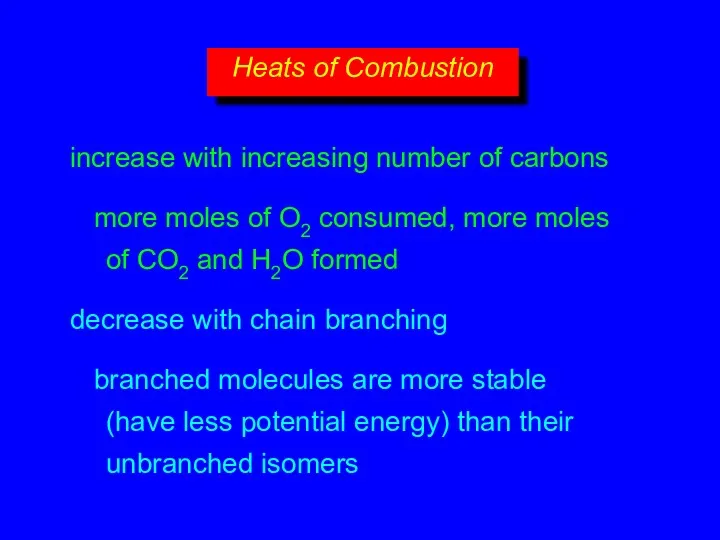
- 15. decrease with chain branching branched molecules are more compact with smaller surface area—fewer points of contact
- 16. 2.15 Chemical Properties. Combustion of Alkanes All alkanes burn in air to give carbon dioxide and
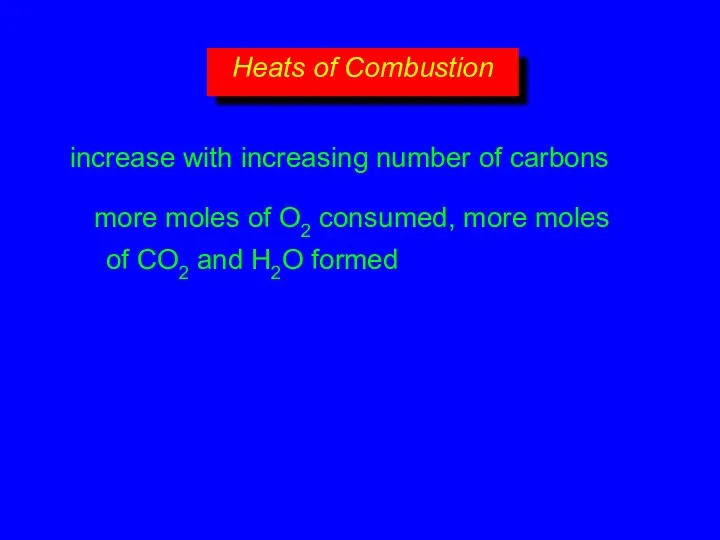
- 17. increase with increasing number of carbons more moles of O2 consumed, more moles of CO2 and
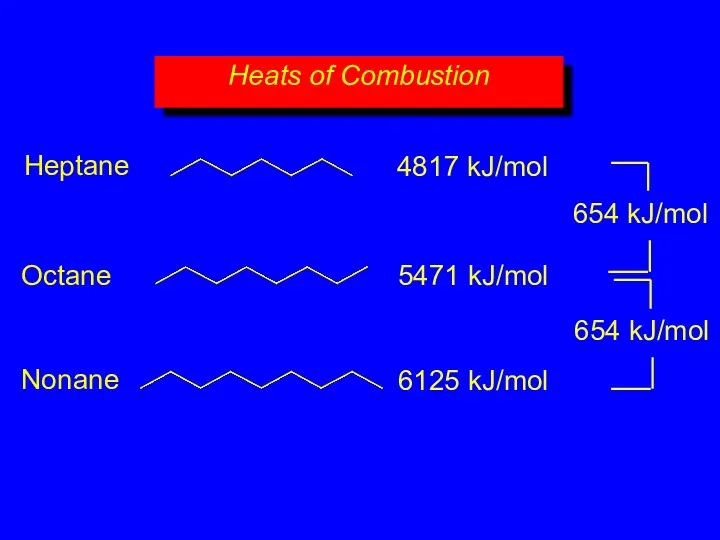
- 18. Heats of Combustion 4817 kJ/mol 5471 kJ/mol 6125 kJ/mol 654 kJ/mol 654 kJ/mol Heptane Octane Nonane
- 19. increase with increasing number of carbons more moles of O2 consumed, more moles of CO2 and
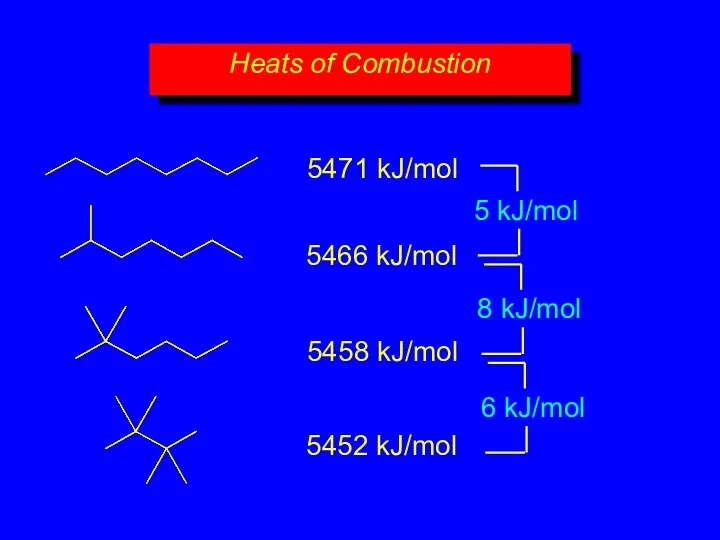
- 20. Heats of Combustion 5471 kJ/mol 5466 kJ/mol 5458 kJ/mol 5452 kJ/mol
- 21. Isomers can differ in respect to their stability. Equivalent statement: Isomers differ in respect to their
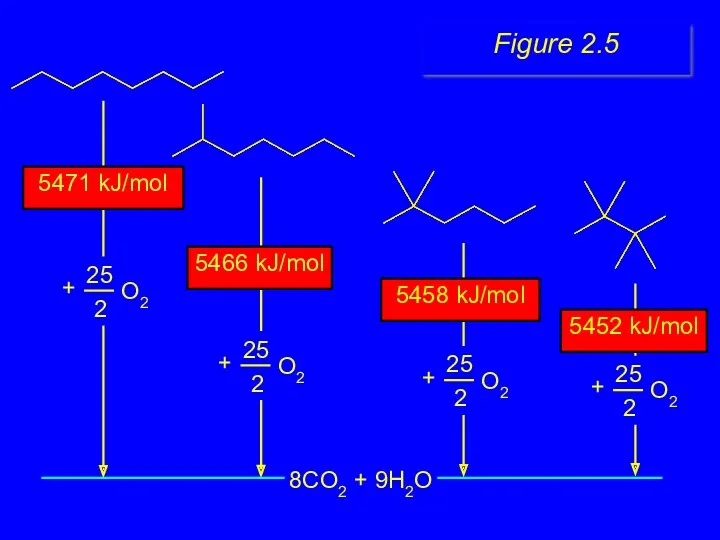
- 22. 8CO2 + 9H2O 5452 kJ/mol 5458 kJ/mol 5471 kJ/mol 5466 kJ/mol Figure 2.5
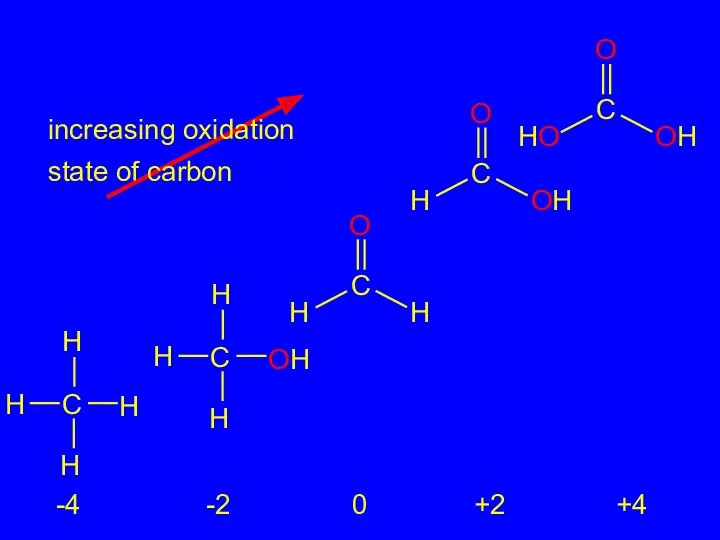
- 23. 2.16 Oxidation-Reduction in Organic Chemistry Oxidation of carbon corresponds to an increase in the number of
- 24. increasing oxidation state of carbon -4 -2 0 +2 +4
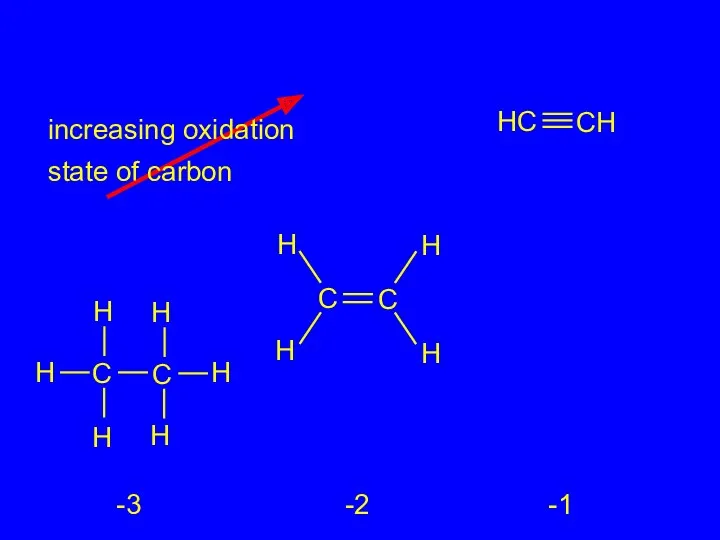
- 25. increasing oxidation state of carbon -3 -2 -1
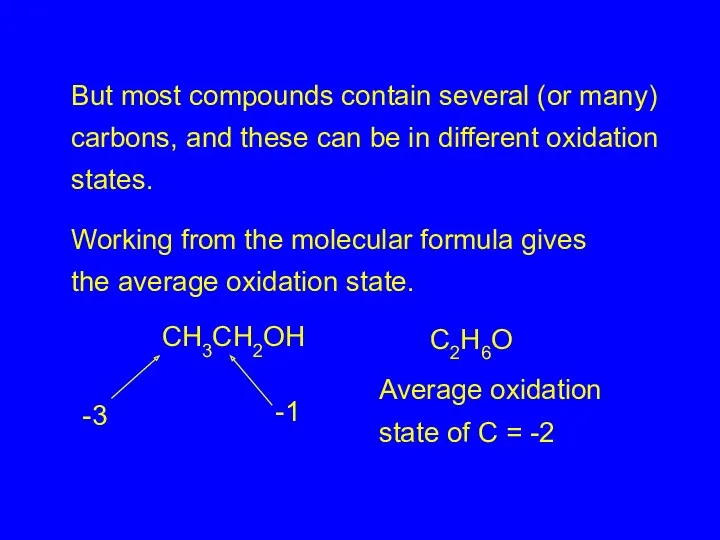
- 26. But most compounds contain several (or many) carbons, and these can be in different oxidation states.
- 27. Fortunately, we rarely need to calculate the oxidation state of individual carbons in a molecule .
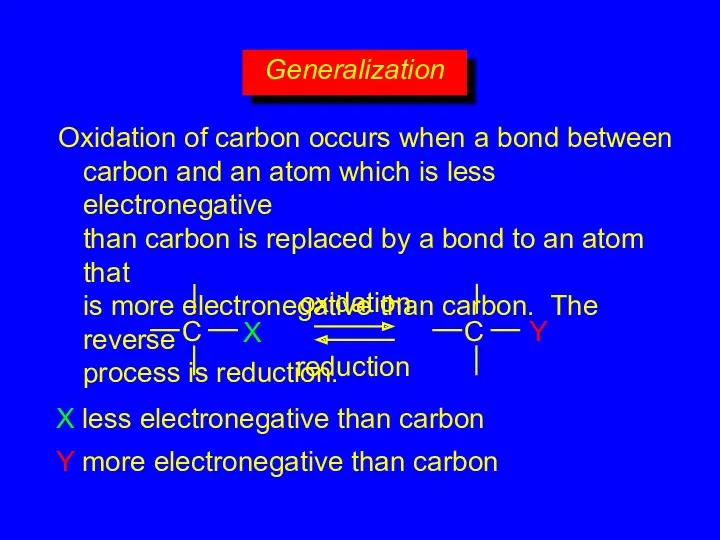
- 28. Generalization Oxidation of carbon occurs when a bond between carbon and an atom which is less
- 30. Скачать презентацию









 Хімічні властивості металів
Хімічні властивості металів Карбоновые кислоты. Тест
Карбоновые кислоты. Тест Материаловедение. Основные задачи материаловедения
Материаловедение. Основные задачи материаловедения Электролитическая диссоциация. Вещества в растворах
Электролитическая диссоциация. Вещества в растворах Общая электронная теория восстановления и окисления металлов
Общая электронная теория восстановления и окисления металлов Фосфор. Урок в 9 классе
Фосфор. Урок в 9 классе Химические свойства кислот
Химические свойства кислот Кристаллические решетки
Кристаллические решетки Пробоотбор других ООС. Лекция 3
Пробоотбор других ООС. Лекция 3 Дисперсные системы в атмосфере
Дисперсные системы в атмосфере Хром. Физические и химические свойства
Хром. Физические и химические свойства Титан және оның қорытпалары. Титанның физика-химиялық қасиеттері
Титан және оның қорытпалары. Титанның физика-химиялық қасиеттері Химический факультет
Химический факультет Химия как компонент системы естественнонаучного образования
Химия как компонент системы естественнонаучного образования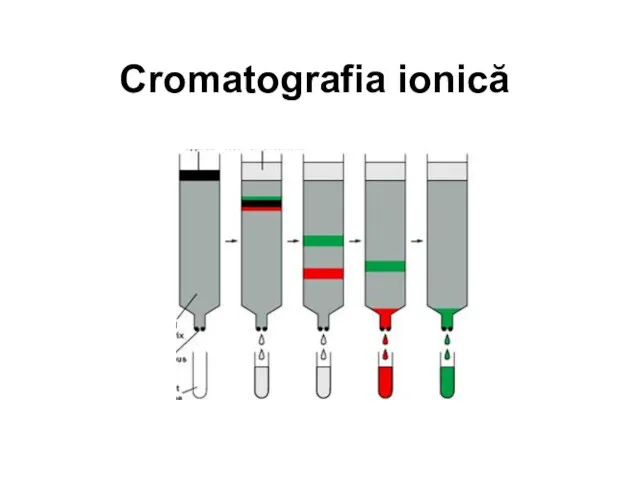 Cromatografia ionică
Cromatografia ionică Основні класи неорганічних сполук
Основні класи неорганічних сполук Алюминий и сплавы на его основе
Алюминий и сплавы на его основе Комплексообразователь. (Лекция 5)
Комплексообразователь. (Лекция 5) Как и где используется соляная кислота
Как и где используется соляная кислота Электрохимические процессы
Электрохимические процессы Кислород. Атом кислорода
Кислород. Атом кислорода Тепловой эффект химических реакций. 8 класс
Тепловой эффект химических реакций. 8 класс Классификация и общая характеристика механизмов образования свободных радикалов и активных форм кислорода
Классификация и общая характеристика механизмов образования свободных радикалов и активных форм кислорода Гранулометрический состав горных пород
Гранулометрический состав горных пород Дезодоранты. Антиперспиранты
Дезодоранты. Антиперспиранты Полимерные растворы. Условия образования
Полимерные растворы. Условия образования Химиялық тепе-теңдік
Химиялық тепе-теңдік Неметаллы. Общая характеристика неметаллов
Неметаллы. Общая характеристика неметаллов