Содержание
- 2. 3) Anionic Polymerization of Polar Monomers - Type of Polar Monomers - Potentiel Problems due to
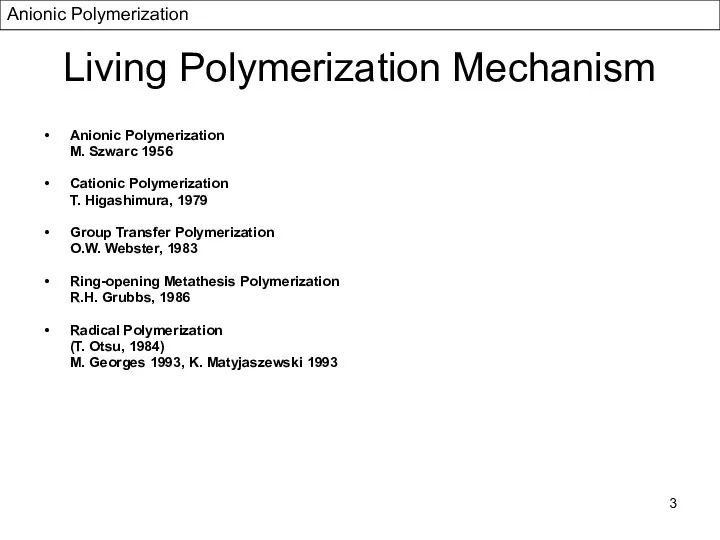
- 3. Living Polymerization Mechanism Anionic Polymerization M. Szwarc 1956 Cationic Polymerization T. Higashimura, 1979 Group Transfer Polymerization
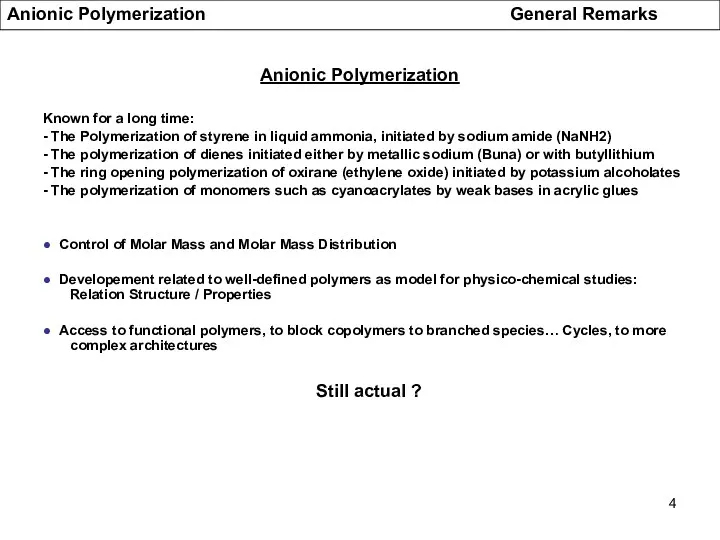
- 4. Anionic Polymerization Known for a long time: - The Polymerization of styrene in liquid ammonia, initiated
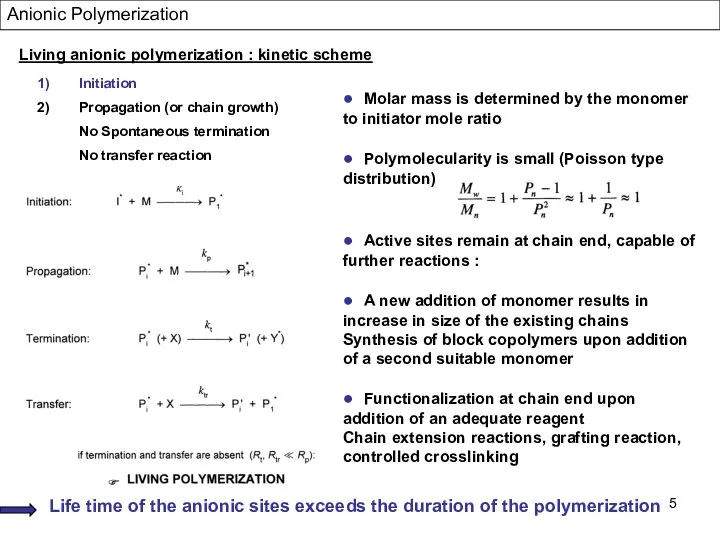
- 5. Living anionic polymerization : kinetic scheme Initiation Propagation (or chain growth) No Spontaneous termination No transfer
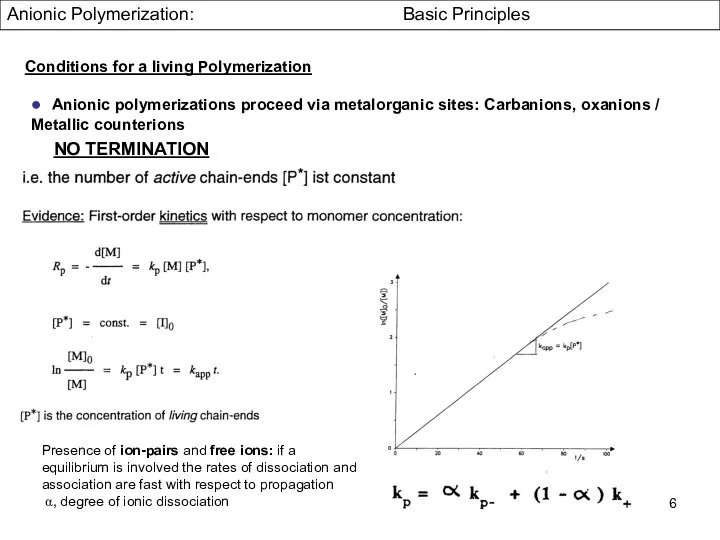
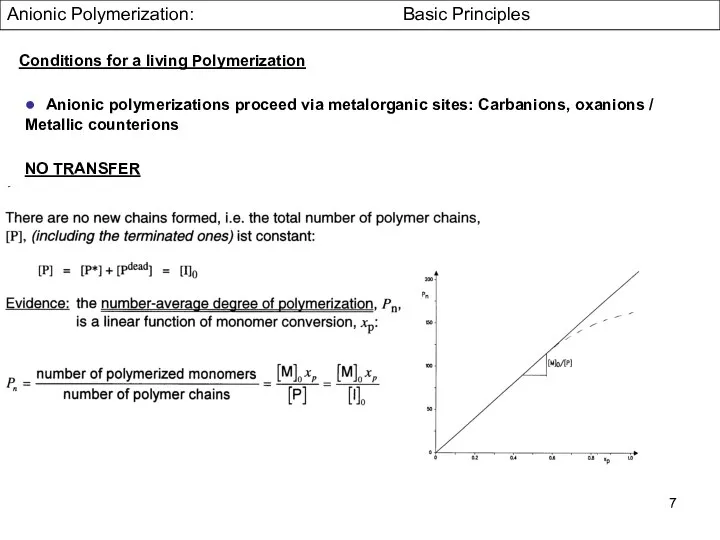
- 6. Anionic Polymerization: Basic Principles ● Anionic polymerizations proceed via metalorganic sites: Carbanions, oxanions / Metallic counterions
- 7. Anionic Polymerization: Basic Principles ● Anionic polymerizations proceed via metalorganic sites: Carbanions, oxanions / Metallic counterions
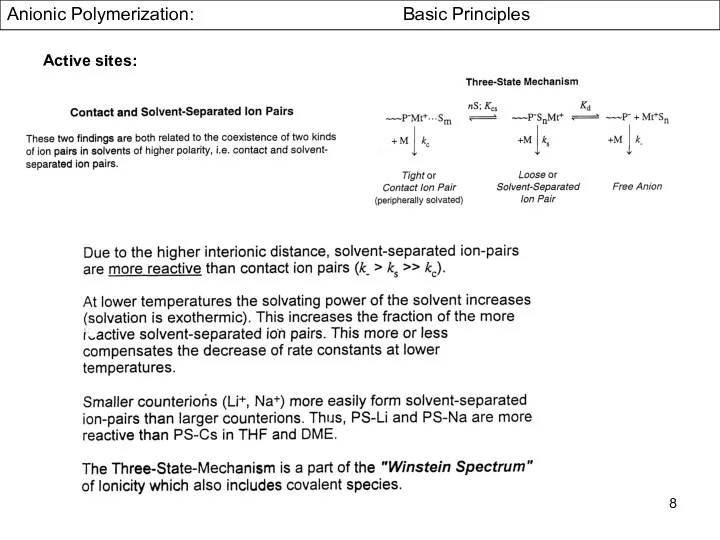
- 8. Anionic Polymerization: Basic Principles Active sites:
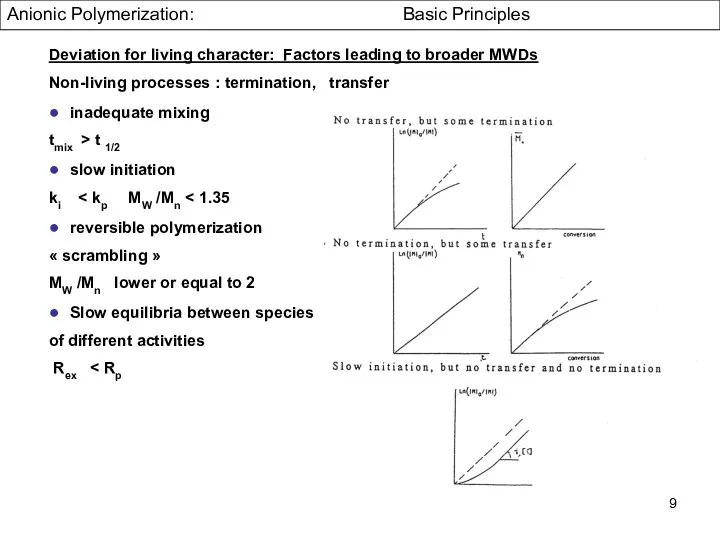
- 9. Deviation for living character: Factors leading to broader MWDs Non-living processes : termination, transfer ● inadequate
- 10. Special consideration for experimental work ● Due to the high nucleophilicity of the initiators (and propagating
- 11. Why is industry interested in living polymerization ? ● Controlled Polymerization Process Predictable Molar Mass Narrow
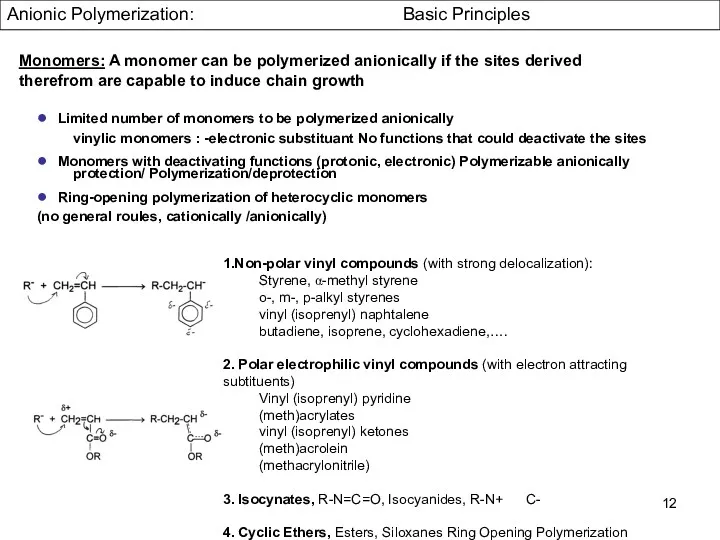
- 12. Monomers: A monomer can be polymerized anionically if the sites derived therefrom are capable to induce
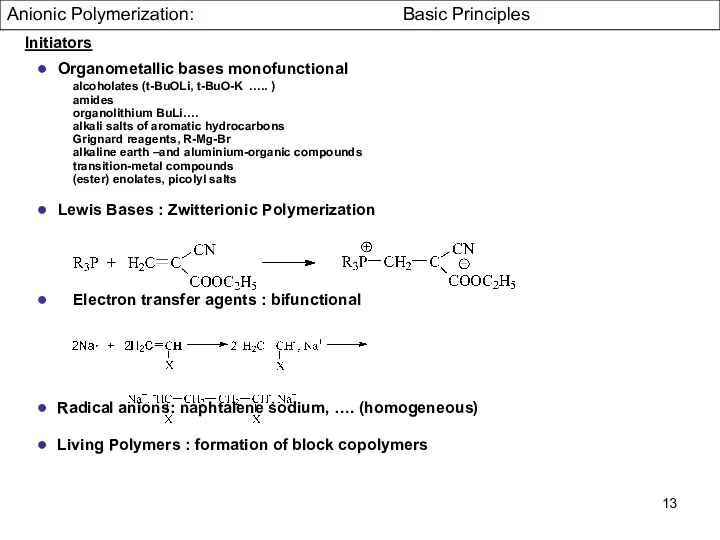
- 13. Initiators ● Organometallic bases monofunctional alcoholates (t-BuOLi, t-BuO-K ….. ) amides organolithium BuLi…. alkali salts of
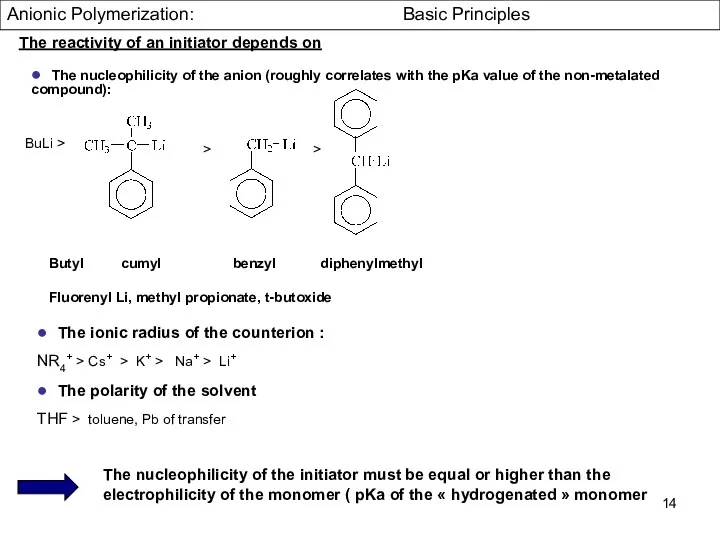
- 14. ● The nucleophilicity of the anion (roughly correlates with the pKa value of the non-metalated compound):
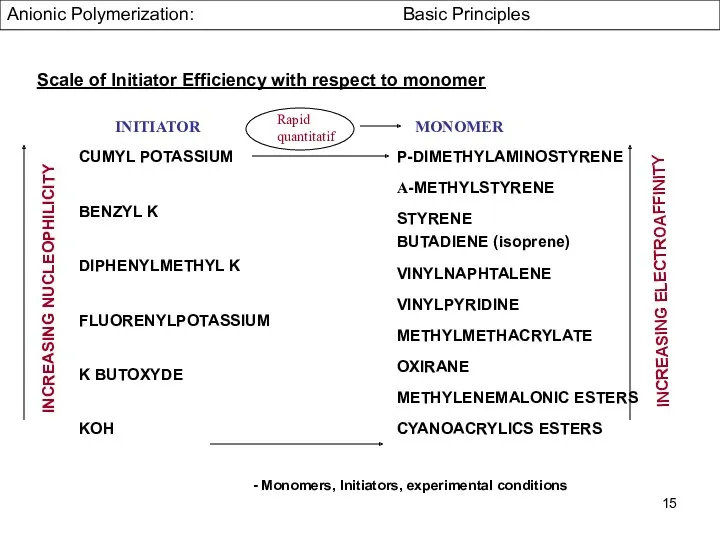
- 15. CUMYL POTASSIUM BENZYL K DIPHENYLMETHYL K FLUORENYLPOTASSIUM K BUTOXYDE KOH INITIATOR MONOMER P-DIMETHYLAMINOSTYRENE Α-METHYLSTYRENE STYRENE BUTADIENE
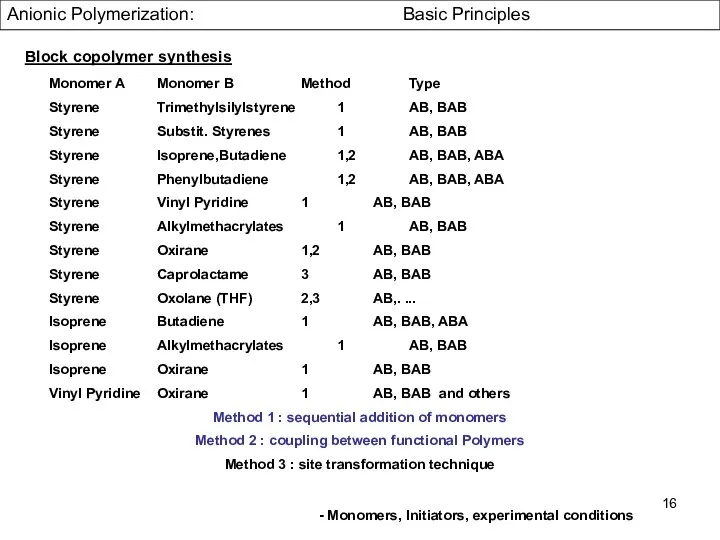
- 16. Monomer A Monomer B Method Type Styrene Trimethylsilylstyrene 1 AB, BAB Styrene Substit. Styrenes 1 AB,
- 17. Anionic Polymerization in Non-Polar Solvents Specific Case of Diene Polymerization of Controlled Microstructure • Non polar
- 18. Structure and Bonding of Organolithium Compounds • Unique compounds : Properties and Characteristics of Covalent compounds
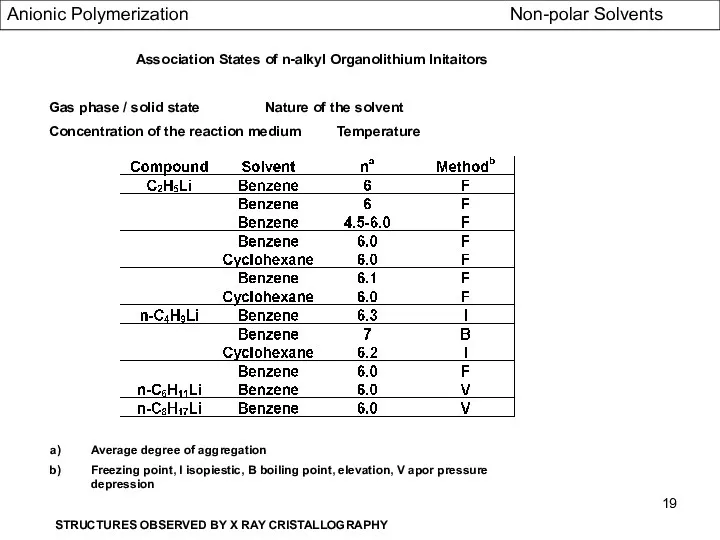
- 19. Association States of n-alkyl Organolithium Initaitors Gas phase / solid state Nature of the solvent Concentration
- 20. • Monofunctional - Soluble in classical non polar solvents - Butyllithium (BuLi) , sec BuLi is
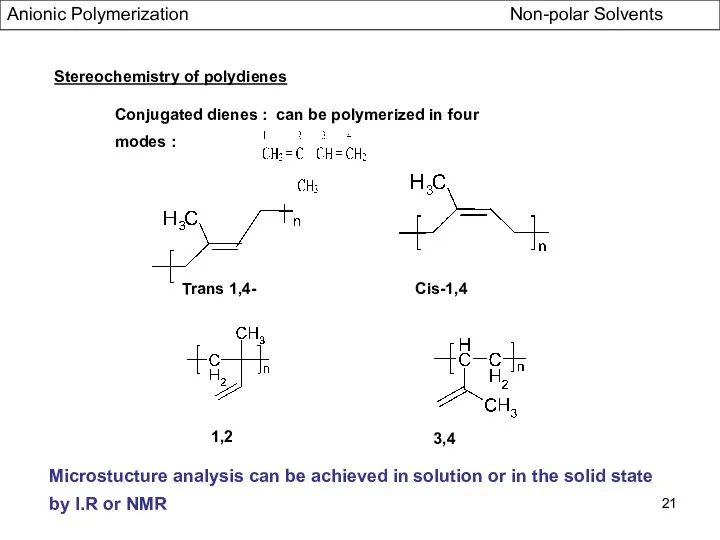
- 21. Stereochemistry of polydienes Conjugated dienes : can be polymerized in four modes : Trans 1,4- Cis-1,4
- 22. Microstructure depends on the - Nature of the counter-ion (Li+, K+, Na+..., Li+ favours 1,4 units
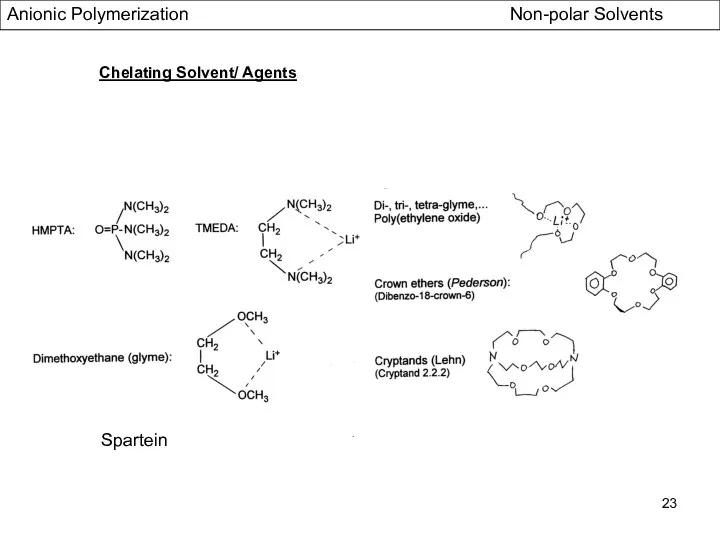
- 23. Chelating Solvent/ Agents Spartein Anionic Polymerization Non-polar Solvents
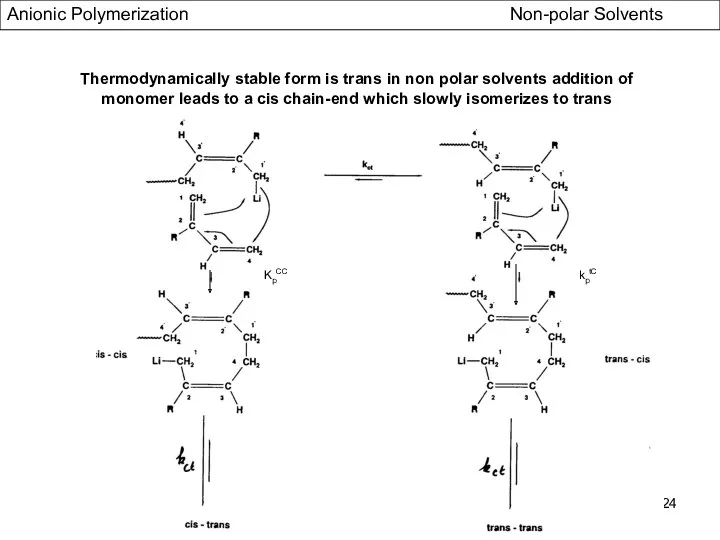
- 24. KpCC kptC Thermodynamically stable form is trans in non polar solvents addition of monomer leads to
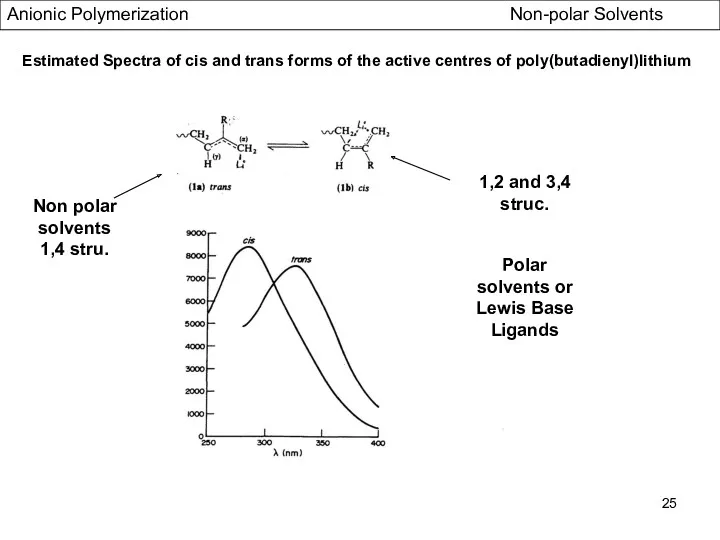
- 25. Estimated Spectra of cis and trans forms of the active centres of poly(butadienyl)lithium Non polar solvents
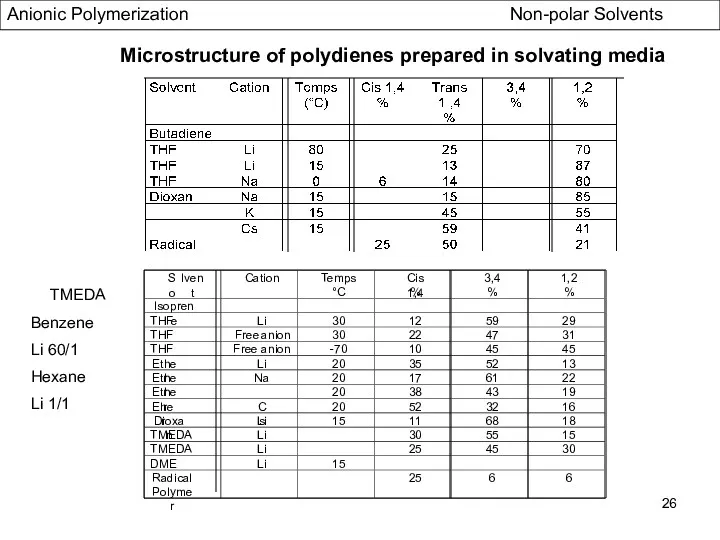
- 26. Microstructure of polydienes prepared in solvating media Radical Polymer 25 6 6 TMEDA Benzene Li 60/1
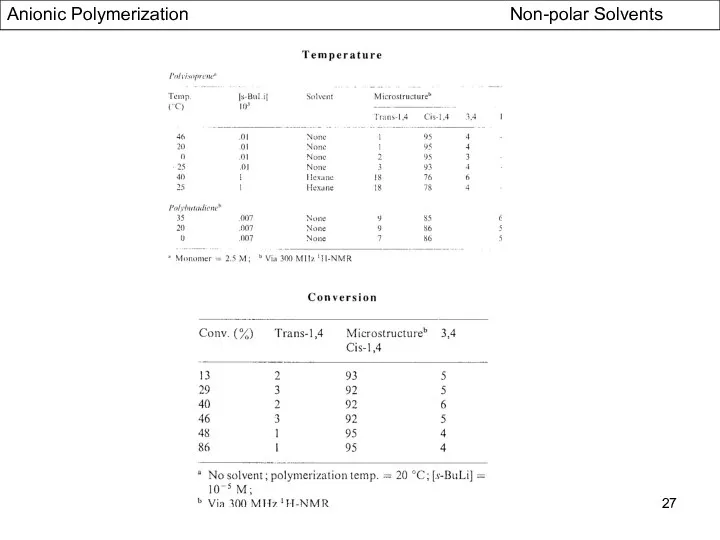
- 27. Anionic Polymerization Non-polar Solvents
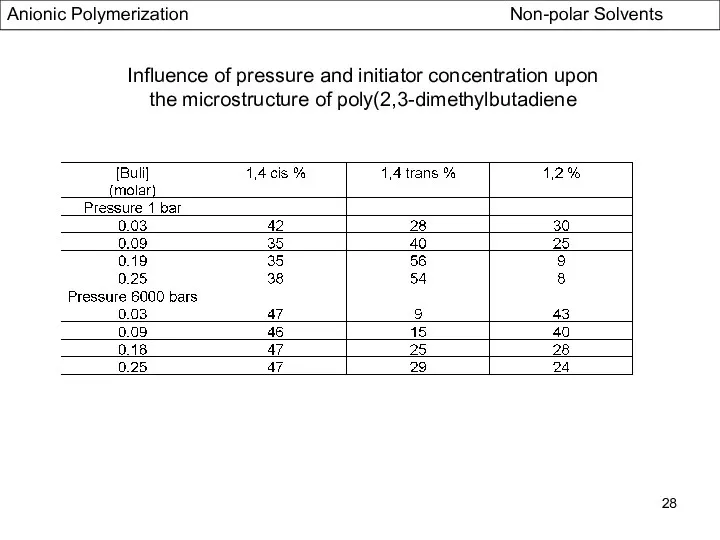
- 28. Influence of pressure and initiator concentration upon the microstructure of poly(2,3-dimethylbutadiene Anionic Polymerization Non-polar Solvents
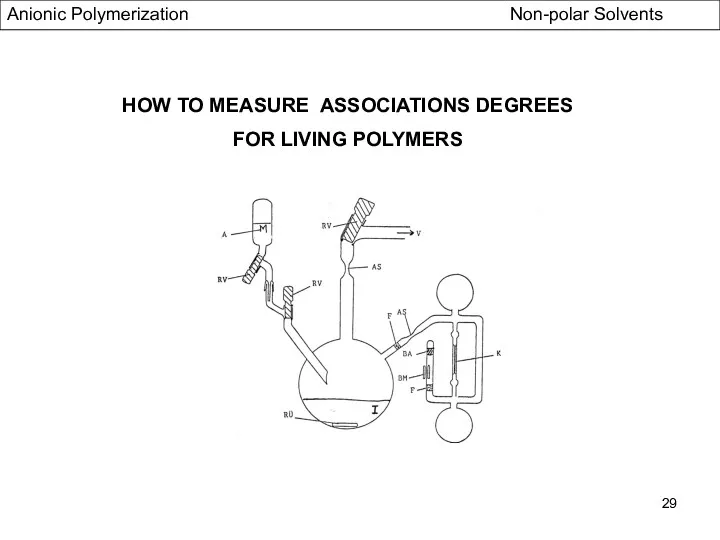
- 29. HOW TO MEASURE ASSOCIATIONS DEGREES FOR LIVING POLYMERS Anionic Polymerization Non-polar Solvents
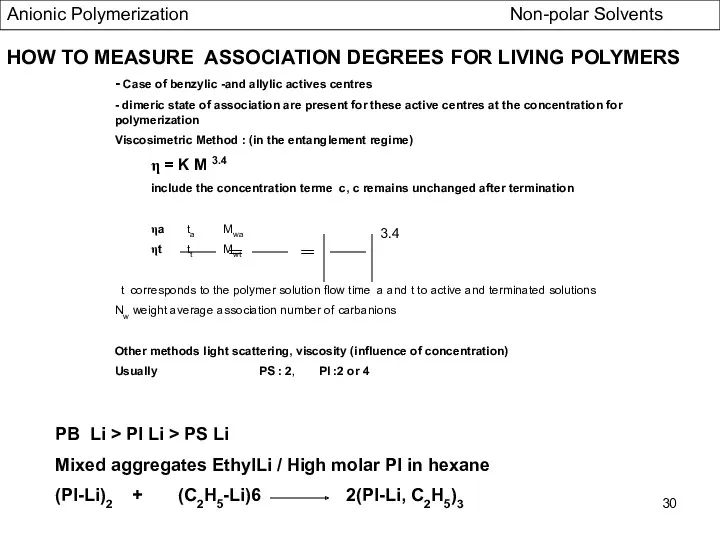
- 30. HOW TO MEASURE ASSOCIATION DEGREES FOR LIVING POLYMERS - Case of benzylic -and allylic actives centres
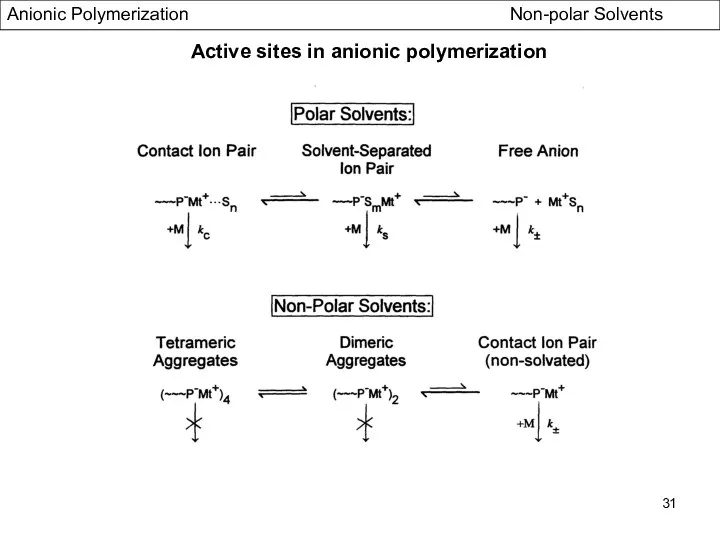
- 31. Active sites in anionic polymerization Anionic Polymerization Non-polar Solvents
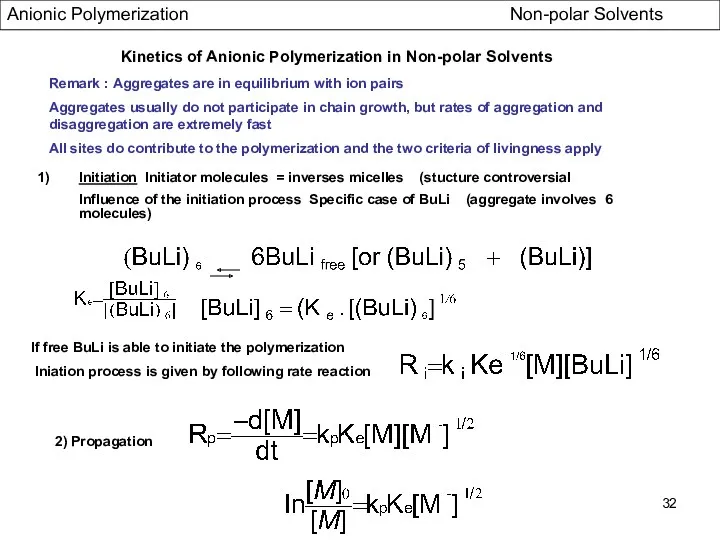
- 32. Kinetics of Anionic Polymerization in Non-polar Solvents Initiation Initiator molecules = inverses micelles (stucture controversial Influence
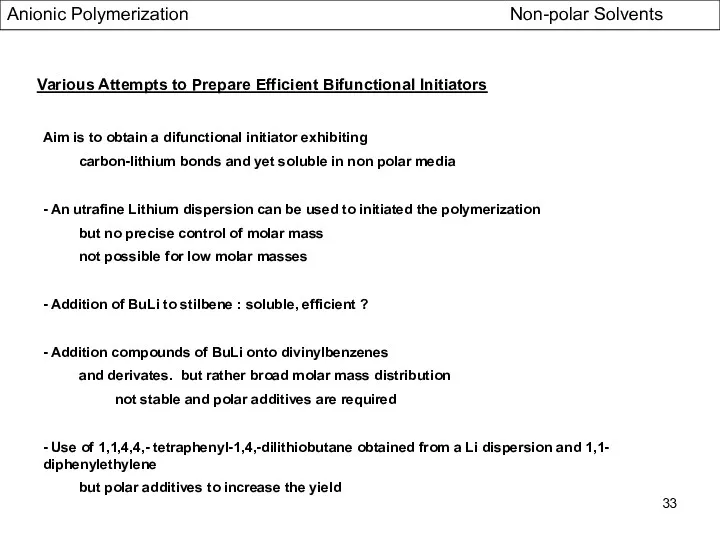
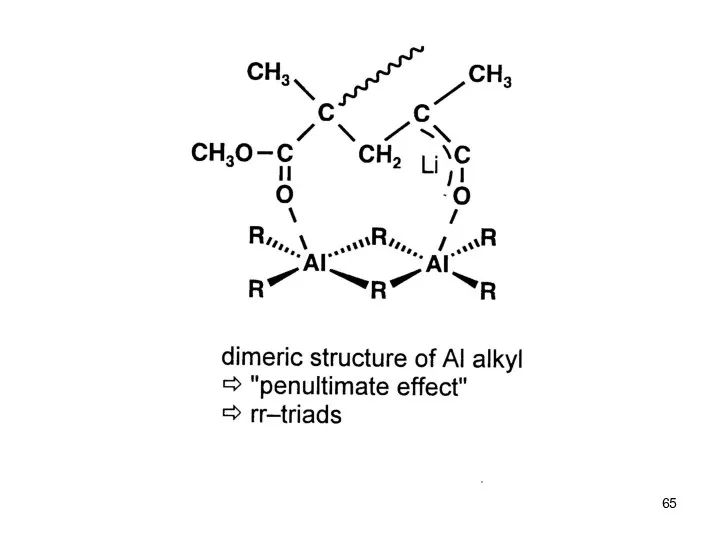
- 33. Various Attempts to Prepare Efficient Bifunctional Initiators Aim is to obtain a difunctional initiator exhibiting carbon-lithium
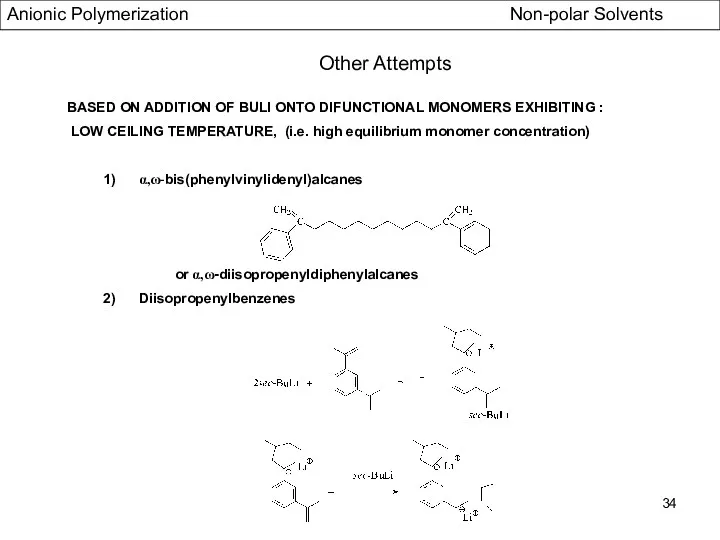
- 34. BASED ON ADDITION OF BULI ONTO DIFUNCTIONAL MONOMERS EXHIBITING : LOW CEILING TEMPERATURE, (i.e. high equilibrium
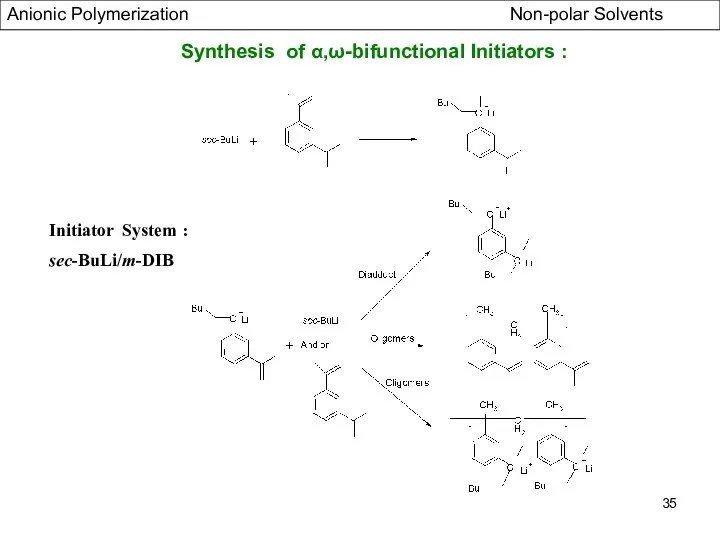
- 35. Synthesis of α,ω-bifunctional Initiators : Initiator System : sec-BuLi/m-DIB Anionic Polymerization Non-polar Solvents
- 36. Diadduct formation sec-BuLi is added at 40° C to DIB ( 1DIB / 2BuLi) under efficient
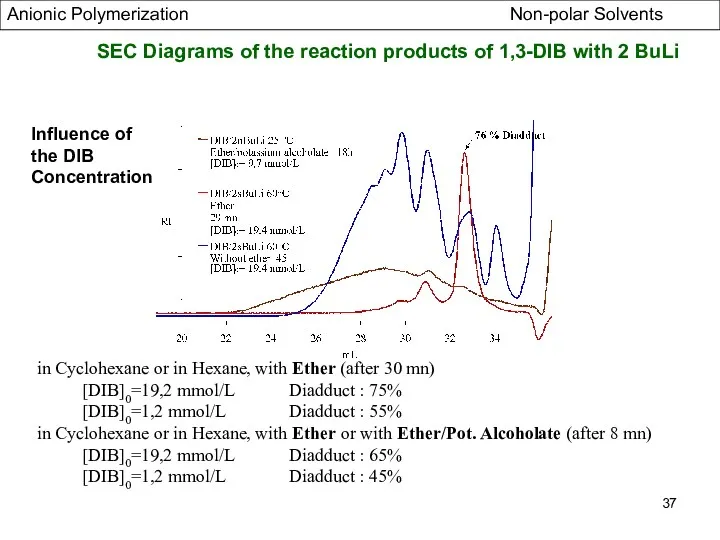
- 37. SEC Diagrams of the reaction products of 1,3-DIB with 2 BuLi Influence of the DIB Concentration
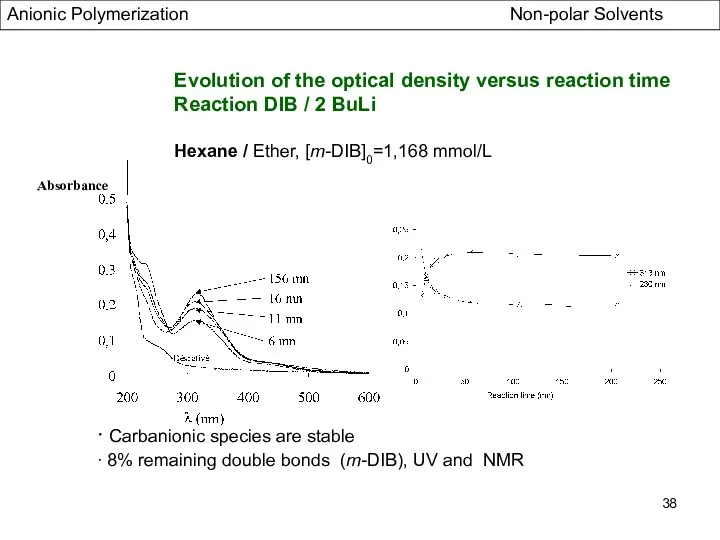
- 38. Absorbance Evolution of the optical density versus reaction time Reaction DIB / 2 BuLi Hexane /
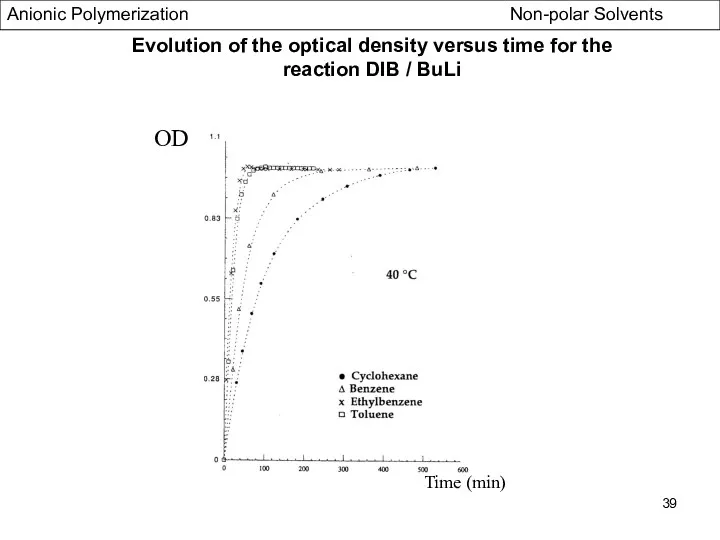
- 39. Time (min) OD Evolution of the optical density versus time for the reaction DIB / BuLi
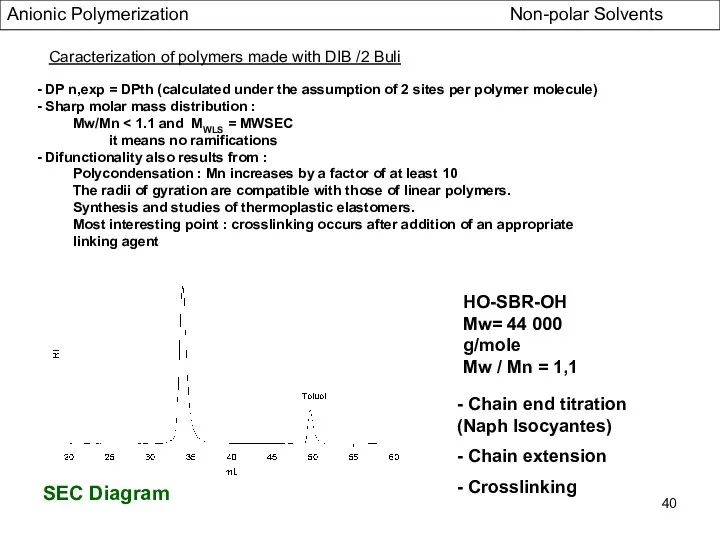
- 40. SEC Diagram HO-SBR-OH Mw= 44 000 g/mole Mw / Mn = 1,1 - Chain end titration
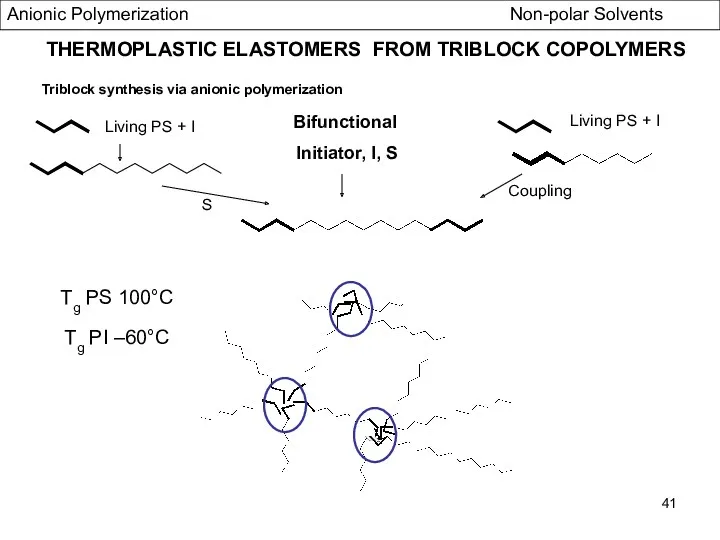
- 41. THERMOPLASTIC ELASTOMERS FROM TRIBLOCK COPOLYMERS Triblock synthesis via anionic polymerization Bifunctional Initiator, I, S Living PS
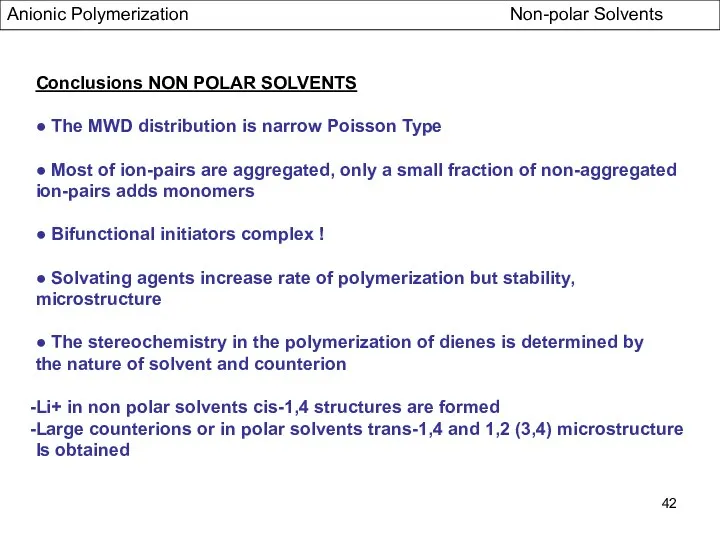
- 42. Anionic Polymerization Non-polar Solvents Conclusions NON POLAR SOLVENTS ● The MWD distribution is narrow Poisson Type
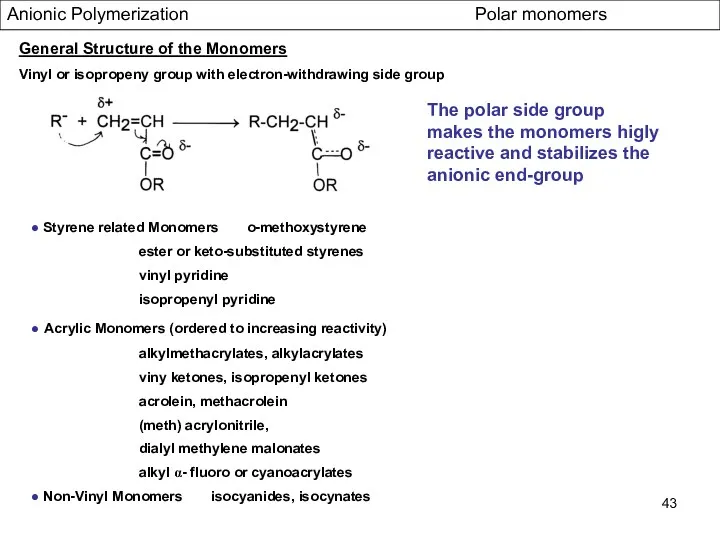
- 43. Anionic Polymerization Polar monomers General Structure of the Monomers Vinyl or isopropeny group with electron-withdrawing side
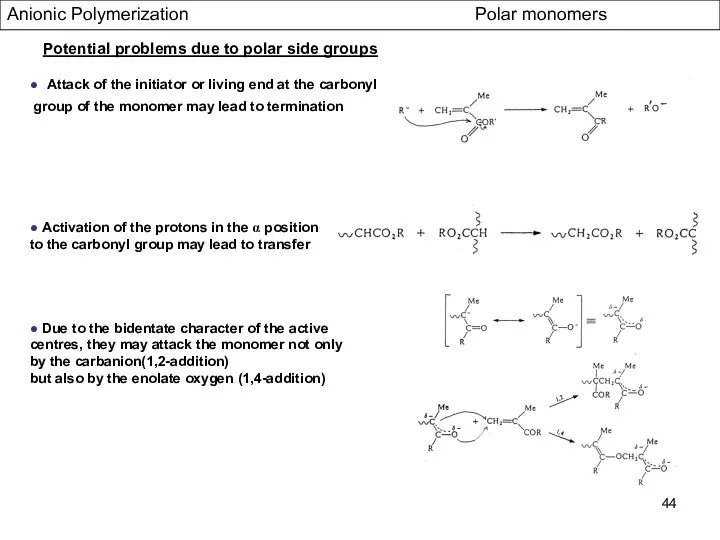
- 44. Anionic Polymerization Polar monomers ● Attack of the initiator or living end at the carbonyl group
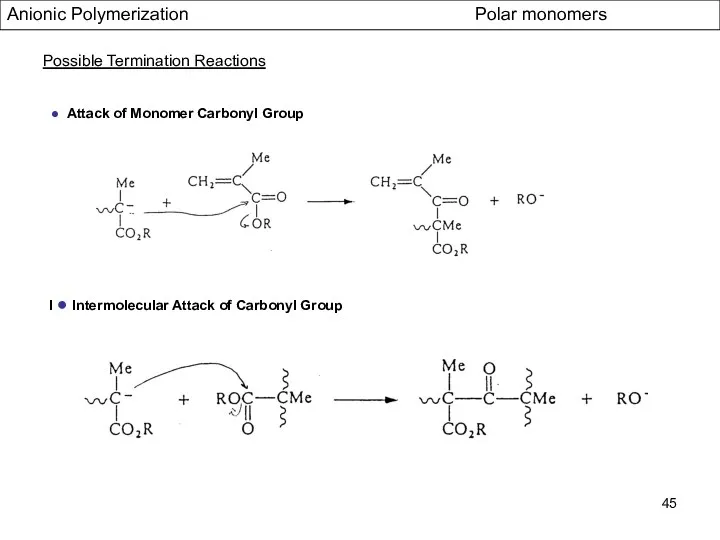
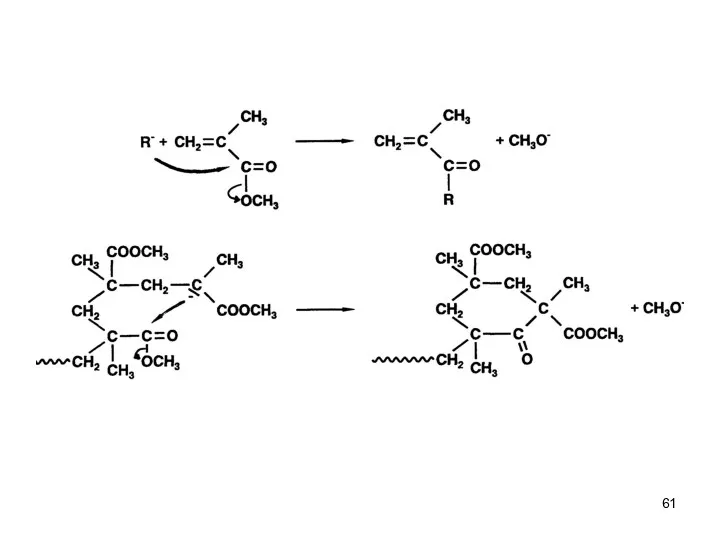
- 45. Anionic Polymerization Polar monomers Possible Termination Reactions ● Attack of Monomer Carbonyl Group I ● Intermolecular
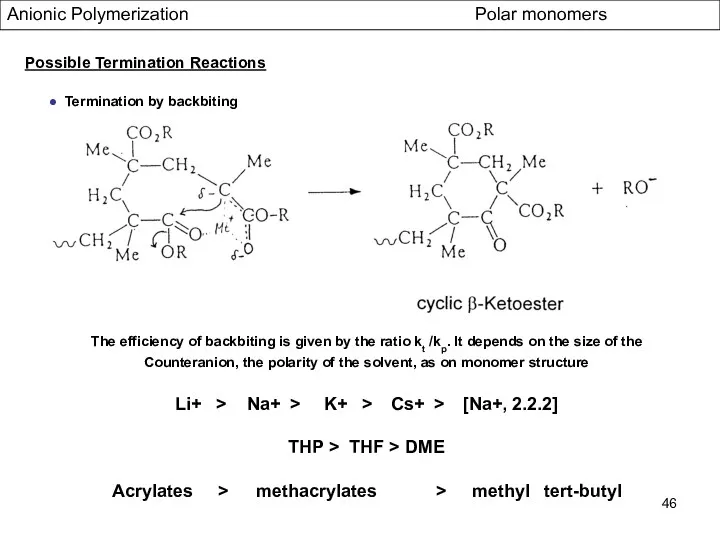
- 46. Anionic Polymerization Polar monomers Possible Termination Reactions ● Termination by backbiting The efficiency of backbiting is
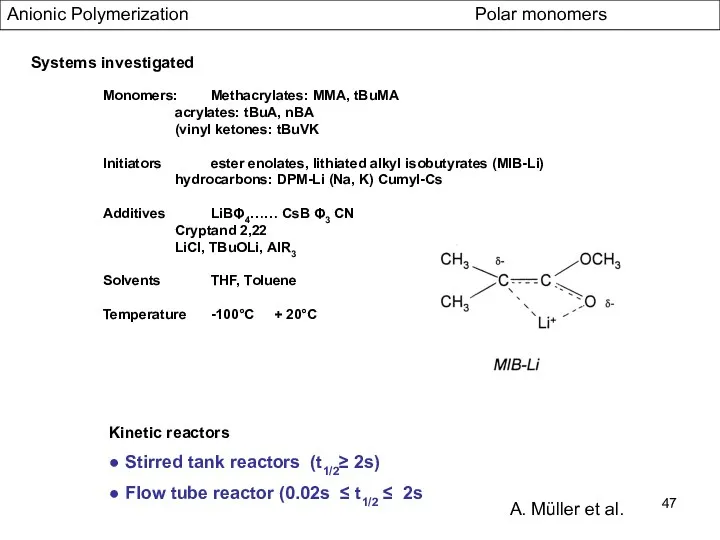
- 47. Anionic Polymerization Polar monomers Systems investigated Monomers: Methacrylates: MMA, tBuMA acrylates: tBuA, nBA (vinyl ketones: tBuVK
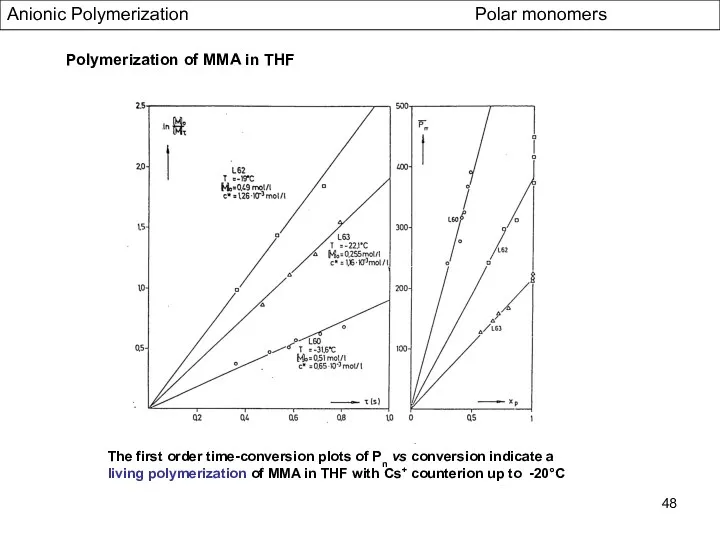
- 48. Anionic Polymerization Polar monomers Polymerization of MMA in THF The first order time-conversion plots of Pn
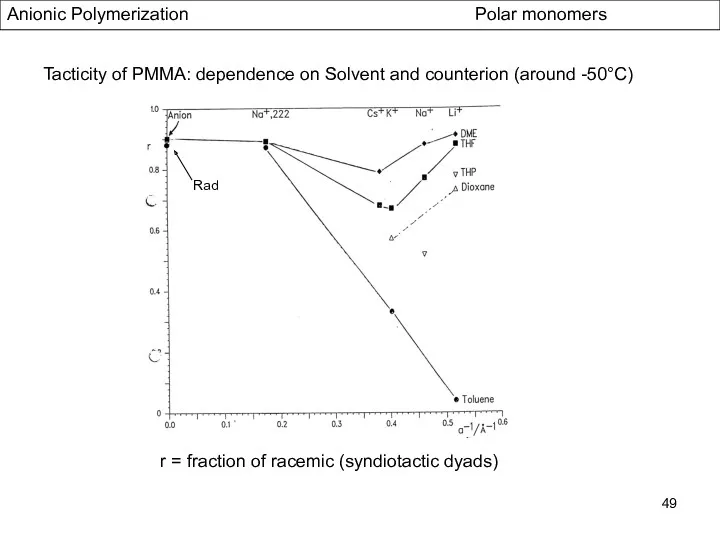
- 49. Anionic Polymerization Polar monomers Tacticity of PMMA: dependence on Solvent and counterion (around -50°C) Rad r
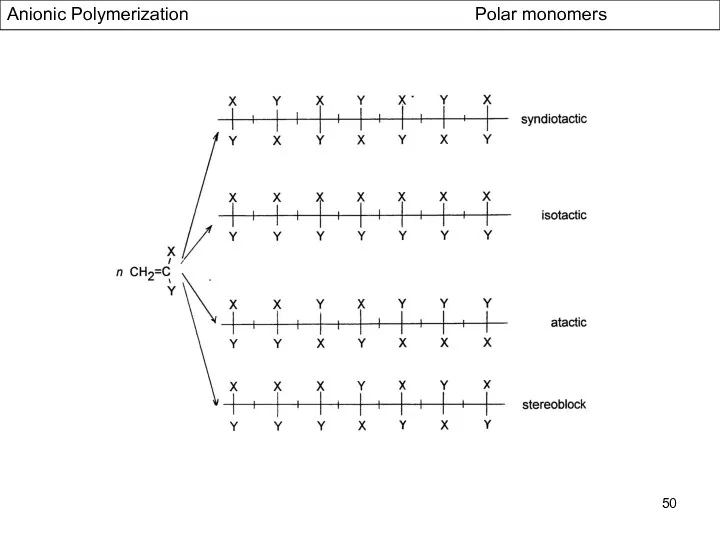
- 50. Anionic Polymerization Polar monomers
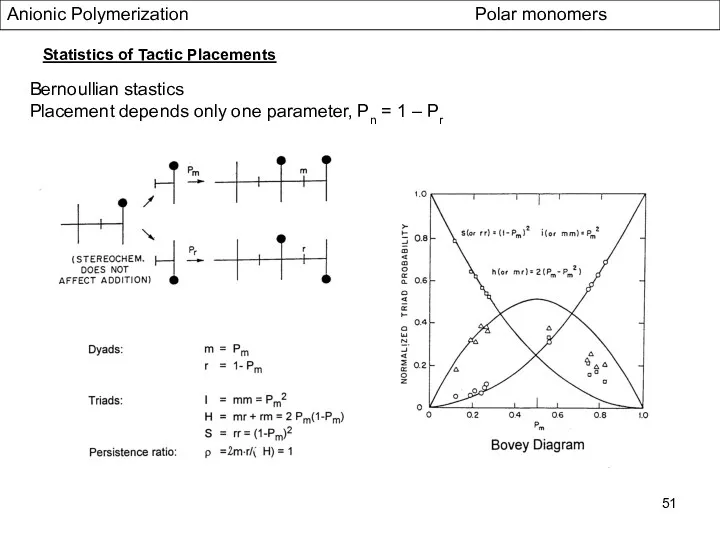
- 51. Anionic Polymerization Polar monomers Statistics of Tactic Placements Bernoullian stastics Placement depends only one parameter, Pn
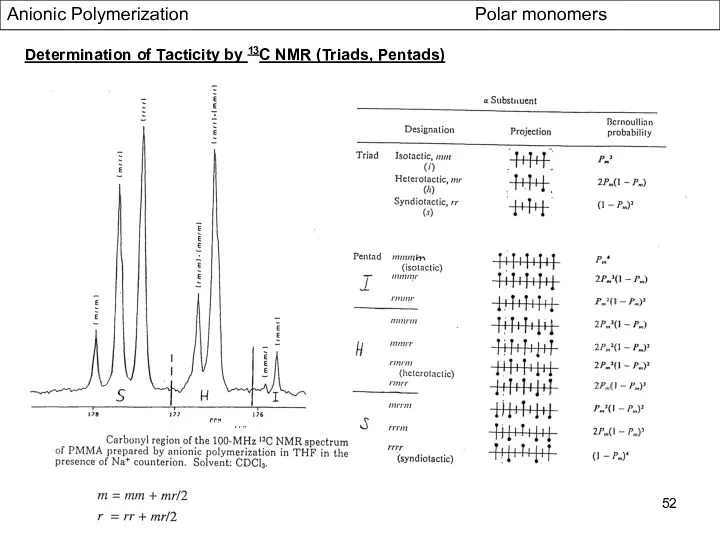
- 52. Anionic Polymerization Polar monomers Determination of Tacticity by 13C NMR (Triads, Pentads)
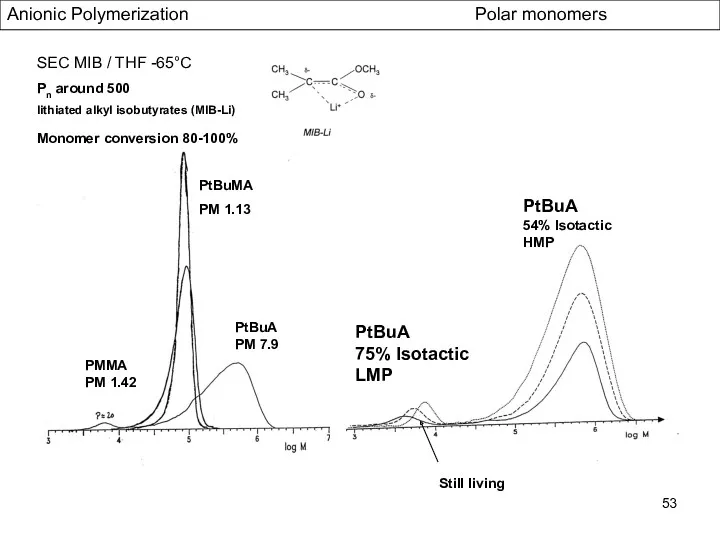
- 53. Anionic Polymerization Polar monomers SEC MIB / THF -65°C Pn around 500 lithiated alkyl isobutyrates (MIB-Li)
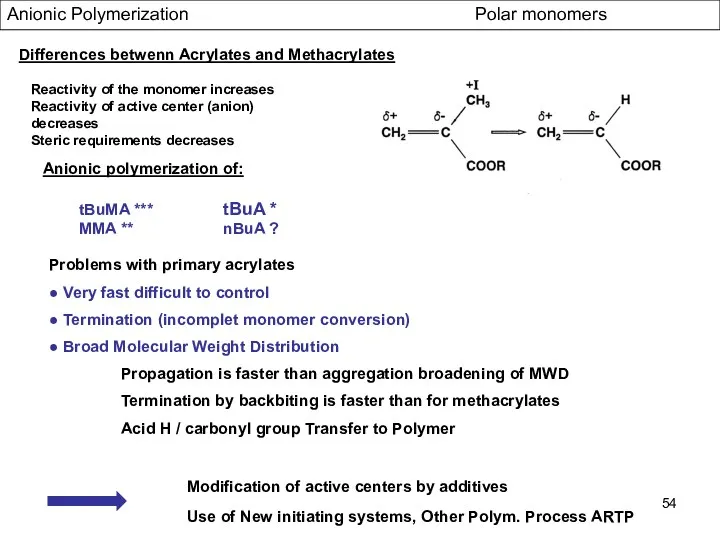
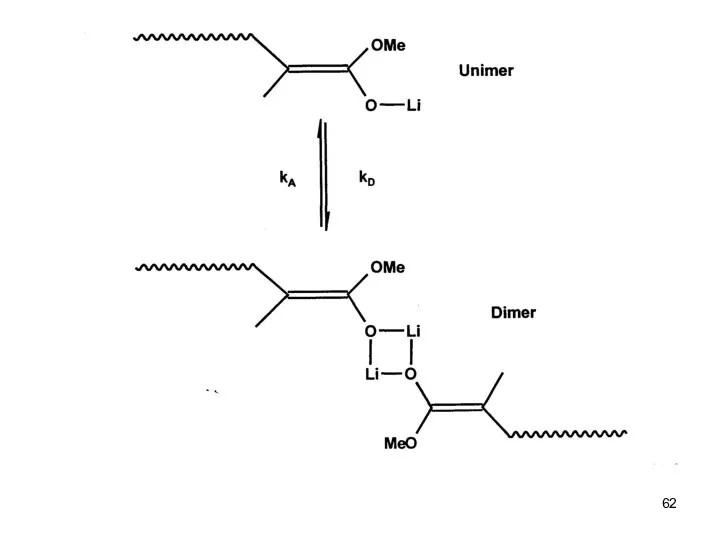
- 54. Anionic Polymerization Polar monomers Differences betwenn Acrylates and Methacrylates Reactivity of the monomer increases Reactivity of
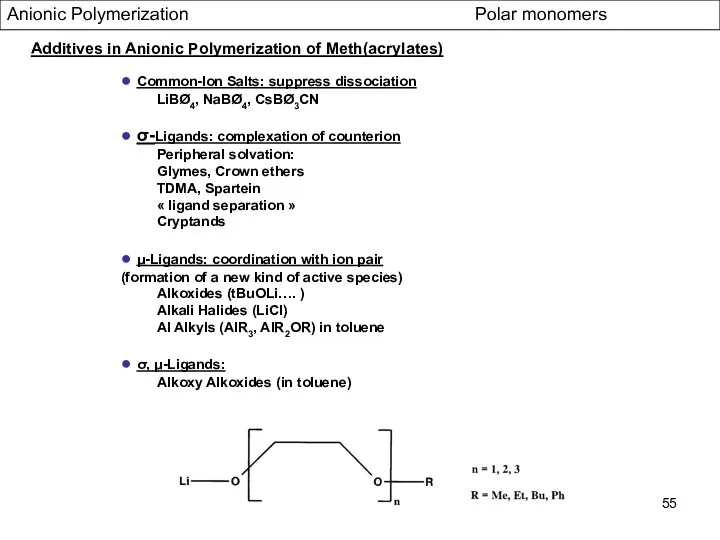
- 55. Anionic Polymerization Polar monomers Additives in Anionic Polymerization of Meth(acrylates) ● Common-Ion Salts: suppress dissociation LiBØ4,
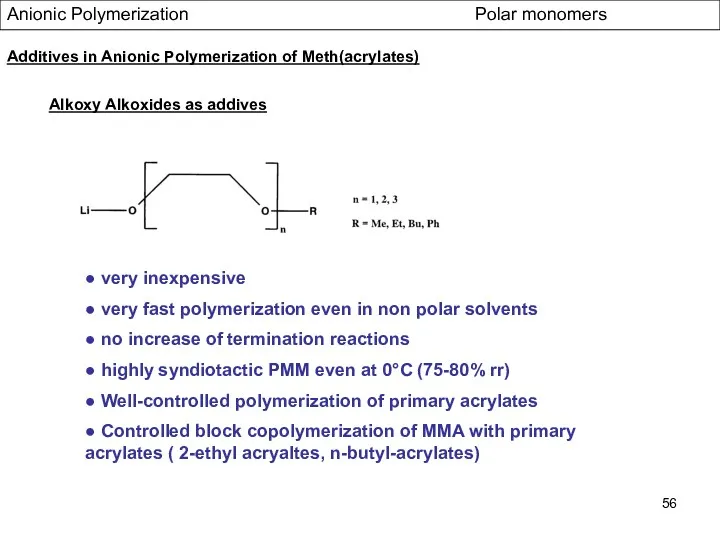
- 56. Alkoxy Alkoxides as addives Anionic Polymerization Polar monomers Additives in Anionic Polymerization of Meth(acrylates) ● very
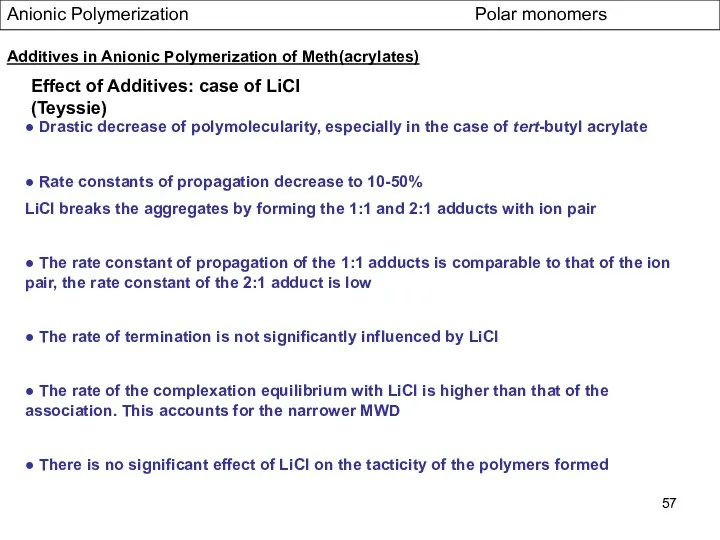
- 57. Anionic Polymerization Polar monomers Effect of Additives: case of LiCl (Teyssie) ● Drastic decrease of polymolecularity,
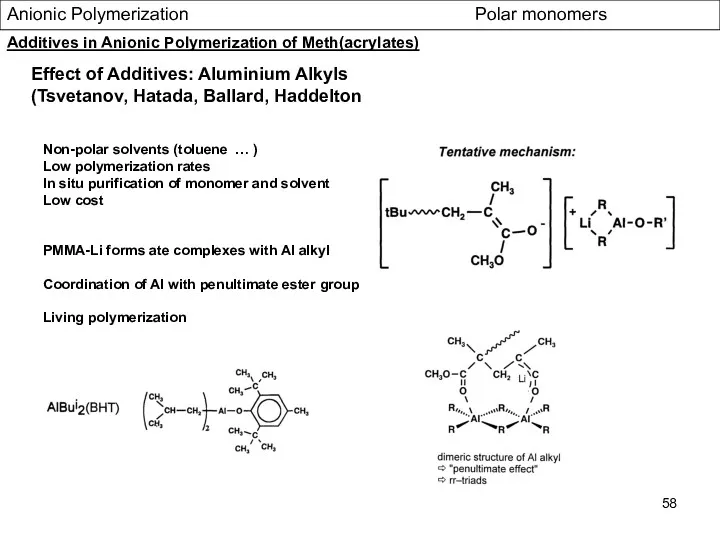
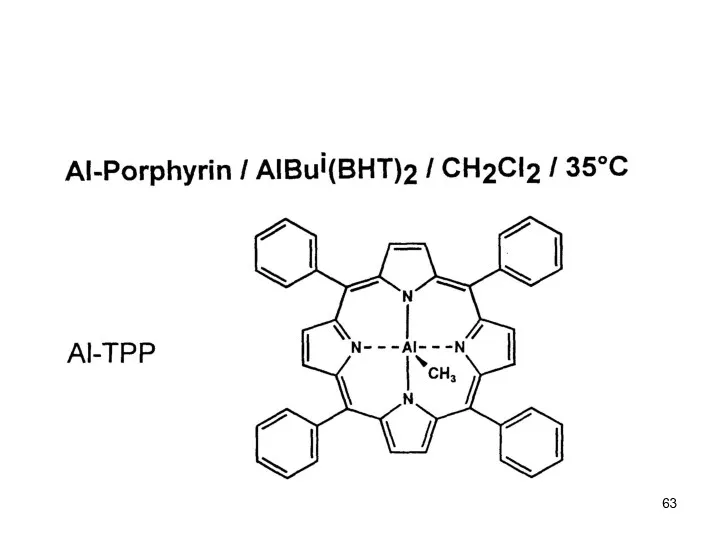
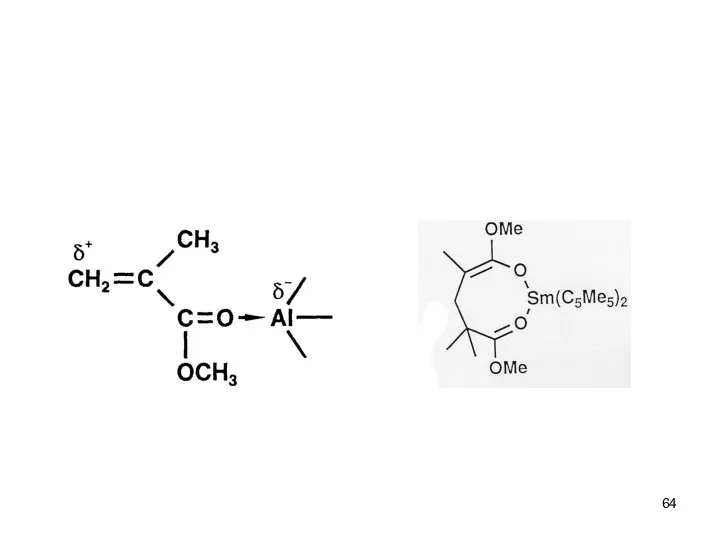
- 58. Anionic Polymerization Polar monomers Effect of Additives: Aluminium Alkyls (Tsvetanov, Hatada, Ballard, Haddelton Non-polar solvents (toluene
- 59. Anionic Polymerization Polar monomers
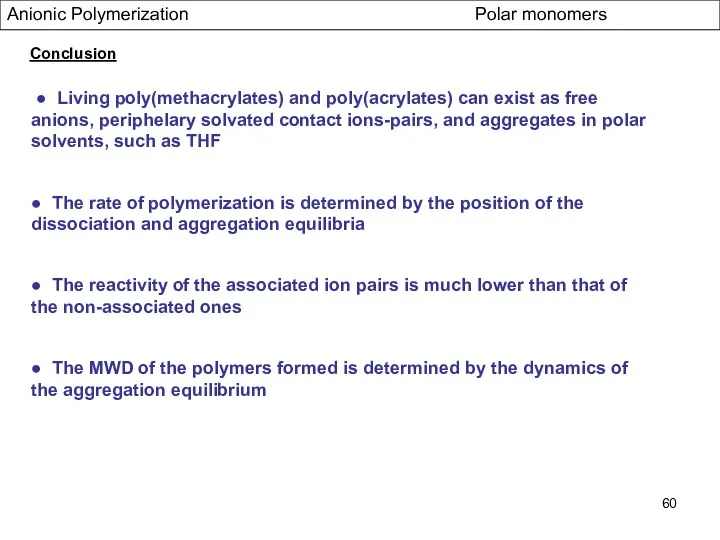
- 60. Anionic Polymerization Polar monomers Conclusion ● Living poly(methacrylates) and poly(acrylates) can exist as free anions, periphelary
- 67. Скачать презентацию






 Массовая доля вещества в растворе
Массовая доля вещества в растворе Состав, строение и свойства натурального каучука
Состав, строение и свойства натурального каучука Study of the properties of halogens and the determination of halide ions in aqueous solution
Study of the properties of halogens and the determination of halide ions in aqueous solution Установка производства серы по методу Клауса
Установка производства серы по методу Клауса Кислород O2
Кислород O2 Литий. Физические и химические свойства. Получение и применение
Литий. Физические и химические свойства. Получение и применение Массообменные процессы
Массообменные процессы Введение в минералогию. Генезис минералов
Введение в минералогию. Генезис минералов Фізичні та хімічні явища (гра)
Фізичні та хімічні явища (гра) Классификация химических реакций в органической химии
Классификация химических реакций в органической химии Кремнекислые породы группа гранитов-риолитов гранодиоритов-дацитов. Интрузивные породы
Кремнекислые породы группа гранитов-риолитов гранодиоритов-дацитов. Интрузивные породы Пурин нуклеозидтері (аденозии 3-фосфор қышқылы, рибоксии). Сапасына Қойылантын талаптар, талдау әдістері
Пурин нуклеозидтері (аденозии 3-фосфор қышқылы, рибоксии). Сапасына Қойылантын талаптар, талдау әдістері История открытия кислорода
История открытия кислорода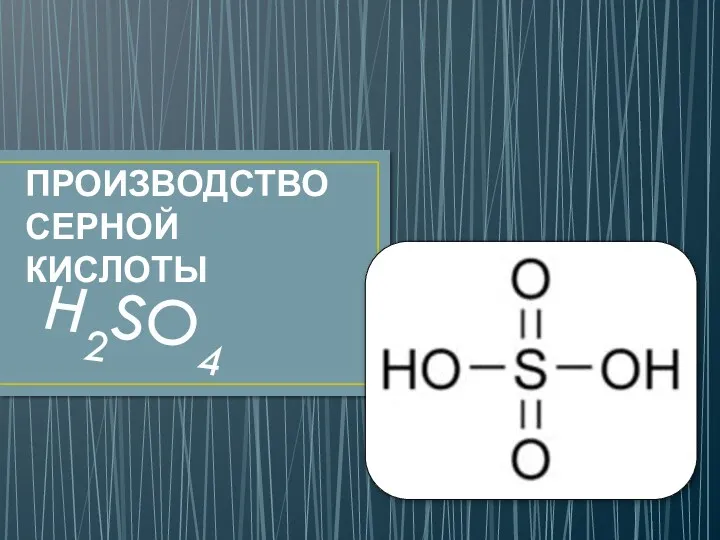 Производство серной кислоты
Производство серной кислоты Основания. (8 класс)
Основания. (8 класс) Хлорид натрия
Хлорид натрия Нефть и нефтепродукты. Происхождение. Состав. Свойства. Переработка
Нефть и нефтепродукты. Происхождение. Состав. Свойства. Переработка История становления органической химии
История становления органической химии Физические явления в химии. Чистые вещества и смеси
Физические явления в химии. Чистые вещества и смеси Строение атома
Строение атома Металловедение. Классификация металлов
Металловедение. Классификация металлов Основные постулаты квантовой механики
Основные постулаты квантовой механики Изохинолин туындыларының дәрілік заттарын талдау
Изохинолин туындыларының дәрілік заттарын талдау Кислоты. Химические свойства кислот
Кислоты. Химические свойства кислот Первоначальные представления об органических веществах. Органическая химия
Первоначальные представления об органических веществах. Органическая химия Изучение физико-химических свойств мицеллярных растворов индивидуальных ПАВ, композиций различных ПАВ
Изучение физико-химических свойств мицеллярных растворов индивидуальных ПАВ, композиций различных ПАВ Природные источники углеводородов
Природные источники углеводородов Відносна густина газів
Відносна густина газів