Study of the properties of halogens and the determination of halide ions in aqueous solution презентация
Содержание
- 2. Outline Introduction Main part 1. General characteristics of halogens 2. Chemical properties of halogens 3. Chlorine
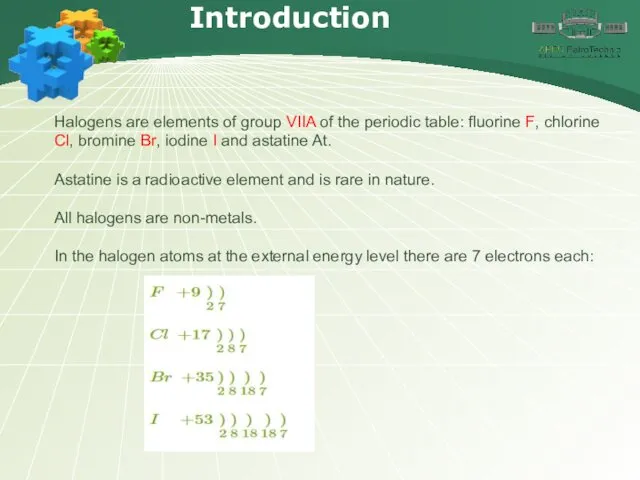
- 4. Introduction Halogens are elements of group VIIA of the periodic table: fluorine F, chlorine Cl, bromine
- 5. Introduction The valence electrons of the halogens form three electron pairs, and one electron of the
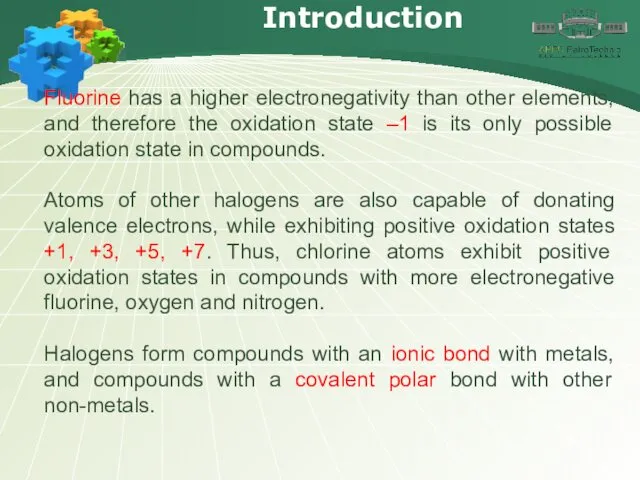
- 6. Introduction Fluorine has a higher electronegativity than other elements, and therefore the oxidation state –1 is
- 7. General characteristics of simple substances Halogen atoms combine in pairs and form diatomic molecules: F2, Cl2,
- 8. General characteristics of simple substances Fluorine Chlorine Bromine Iodine
- 9. General characteristics of simple substances When heated, solid iodine easily sublimes (goes into a gaseous state
- 10. General characteristics of simple substances All halogens have a strong, unpleasant odor and are highly toxic.
- 11. 2. Chemical properties of halogens Halogens are reactive substances. In reactions with metals and most non-metals,
- 12. 2. Chemical properties of halogens Interaction with metals When halogens interact with metals, salts are formed:
- 13. 2. Chemical properties of halogens Interaction with hydrogen In the reactions of halogens with hydrogen, gaseous
- 14. 2. Chemical properties of halogens The reaction of iodine with hydrogen is slow, even when heated.
- 15. 2. Chemical properties of halogens The each other displacements of Halogens from salts In the reactions
- 16. 2. Chemical properties of halogens Bromine is able to displace iodine from iodides, but does not
- 17. 3. Chlorine and its compounds Chlorine Chlorine is a poisonous, yellow-green gas with an unpleasant odor.
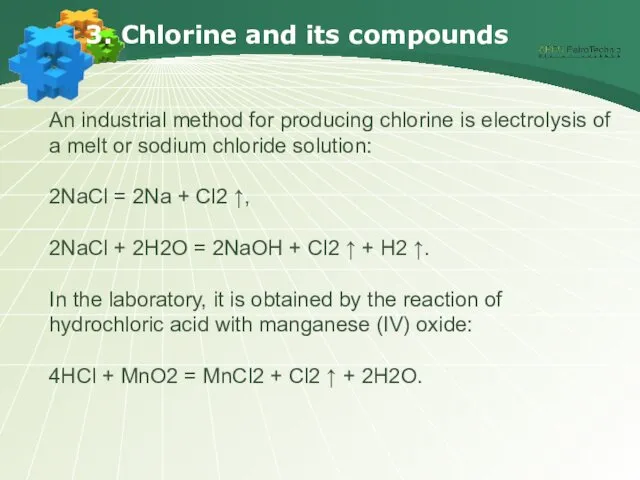
- 18. 3. Chlorine and its compounds An industrial method for producing chlorine is electrolysis of a melt
- 19. 3. Chlorine and its compounds Hydrogen chloride Hydrogen chloride is formed by the interaction of chlorine
- 20. 3. Chlorine and its compounds Hydrochloric acid A solution of hydrogen chloride in water is called
- 21. 3. Chlorine and its compounds interacts with bases and amphoteric hydroxides: KOH + HCl = H2O
- 22. 3. Chlorine and its compounds Chlorides Most hydrochloric acid salts are readily soluble in water. Silver
- 23. 4. Halogens in nature. The use of halogens and their compounds Halogens in nature Halogens are
- 24. 4. Halogens in nature. The use of halogens and their compounds The most common chlorine compounds
- 25. 4. Halogens in nature. The use of halogens and their compounds Bromine and iodine do not
- 26. 4. Halogens in nature. The use of halogens and their compounds Halogens in living organisms All
- 27. 4. Halogens in nature. The use of halogens and their compounds Bromine compounds regulate the processes
- 28. 4. Halogens in nature. The use of halogens and their compounds The use of halogens and
- 29. 4. Halogens in nature. The use of halogens and their compounds Molecular chlorine is used for
- 30. 4. Halogens in nature. The use of halogens and their compounds Table salt is added to
- 31. 1.Cl2 reacts with substance (s): A)CuCl2 B)LiI C)CuBr2 D)FeF3 2.Note the property of hydrogen chloride: A)forms
- 32. 4.In the series F - Cl - Br - I, it decreases: A)number of protons in
- 33. 8.All substances of the series enter into a reaction with hydrochloric acid: A)Ag, Cu, Au B)Ca(OH)2,
- 35. Скачать презентацию


























 Кислоты. Растворы всех кислот
Кислоты. Растворы всех кислот Хімія та їжа
Хімія та їжа Химическая связь
Химическая связь Литий
Литий Классификация органических соединений
Классификация органических соединений Гидролиз солей
Гидролиз солей Аминокислоты. Пептиды. Белки
Аминокислоты. Пептиды. Белки Кремний и его соединения
Кремний и его соединения Гідроліз солей
Гідроліз солей Периодический закон Менделеева
Периодический закон Менделеева Изучение строения и свойств глюкозы
Изучение строения и свойств глюкозы Минералы. Принципы классификации минералов
Минералы. Принципы классификации минералов Лекция 6. Электрофильное присоединение к кратным связям
Лекция 6. Электрофильное присоединение к кратным связям Прикладные аспекты химии поверхностно-активных веществ
Прикладные аспекты химии поверхностно-активных веществ Фенолы
Фенолы Физико-химические процессы в системе свинец - сталь - кислород, для энергетических ядерных реакторов
Физико-химические процессы в системе свинец - сталь - кислород, для энергетических ядерных реакторов Теория строения органических соединений А.М. Бутлерова
Теория строения органических соединений А.М. Бутлерова Катионная полимеризация (Лекция 6)
Катионная полимеризация (Лекция 6) Теорія сильних і слабких електролітів. Рівновага в розчинах малорозчинних електролітів
Теорія сильних і слабких електролітів. Рівновага в розчинах малорозчинних електролітів Алюминий. Строение и свойство атомов
Алюминий. Строение и свойство атомов Методы исследования гидрохимического режима водоемов
Методы исследования гидрохимического режима водоемов Алкадиены
Алкадиены Кремний и его соединения. Простое вещество - кристаллический кремний Si
Кремний и его соединения. Простое вещество - кристаллический кремний Si Аналитическая химия. Предмет и задачи
Аналитическая химия. Предмет и задачи Вода. Практическая работа
Вода. Практическая работа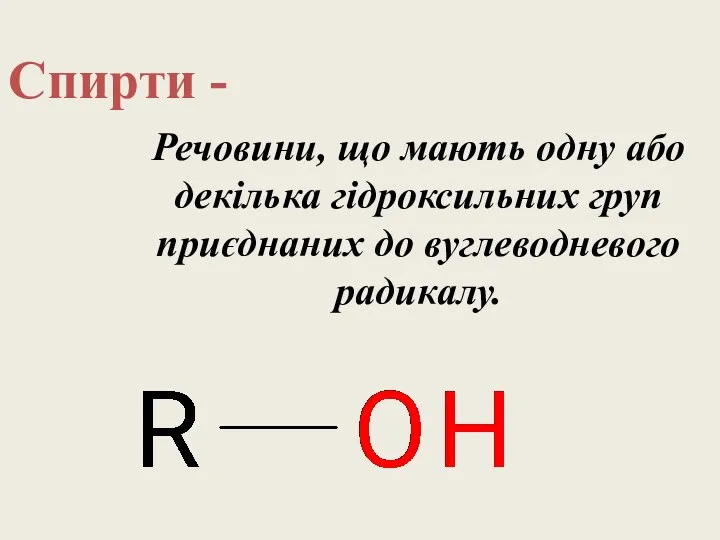 Спирти
Спирти Электролитическая диссоциация. Вещества в растворах
Электролитическая диссоциация. Вещества в растворах Твердое состояние вещества. Плавление
Твердое состояние вещества. Плавление