Содержание
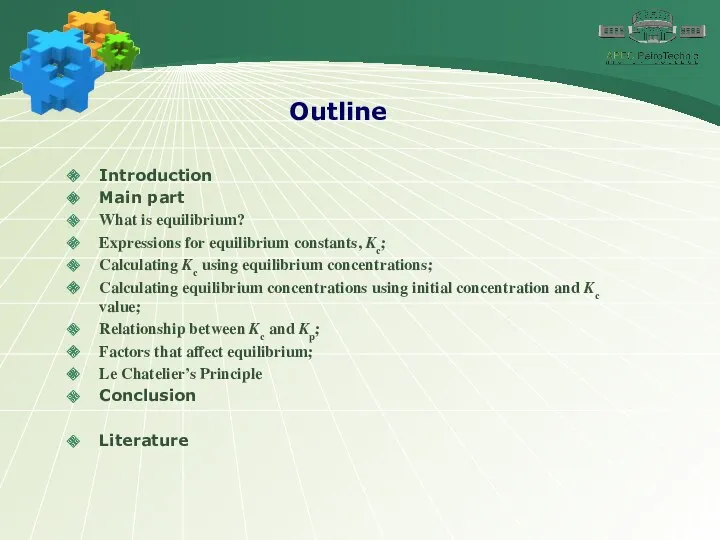
- 2. Outline Introduction Main part What is equilibrium? Expressions for equilibrium constants, Kc; Calculating Kc using equilibrium
- 3. What is Equilibrium?
- 4. This is not Equilibrium?
- 5. Chemical Equilibrium in Nature: (The formation of stalagmites and Stalactites)
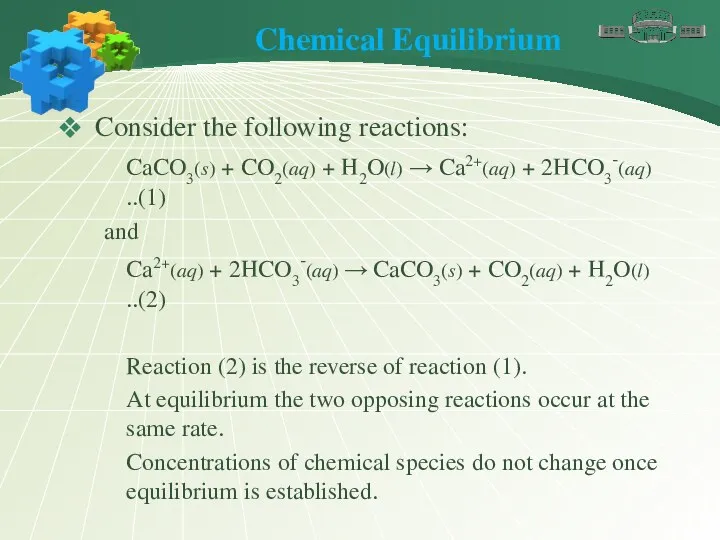
- 6. Chemical Equilibrium Consider the following reactions: CaCO3(s) + CO2(aq) + H2O(l) → Ca2+(aq) + 2HCO3-(aq) ..(1)
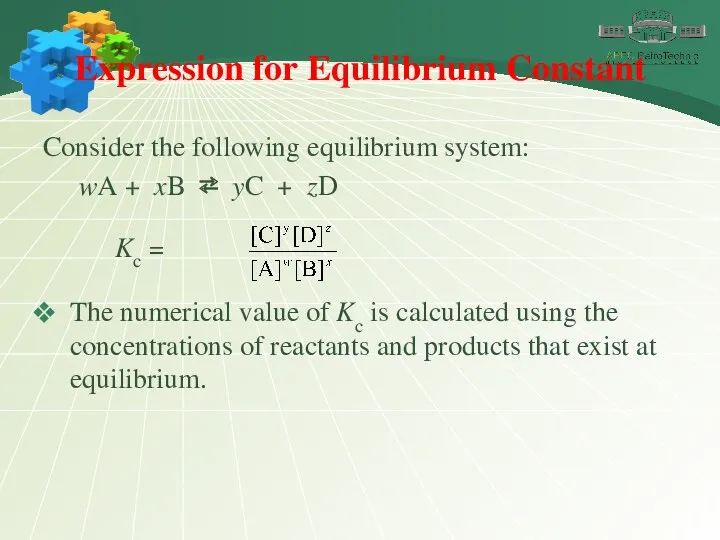
- 7. Expression for Equilibrium Constant Consider the following equilibrium system: wA + xB ⇄ yC + zD
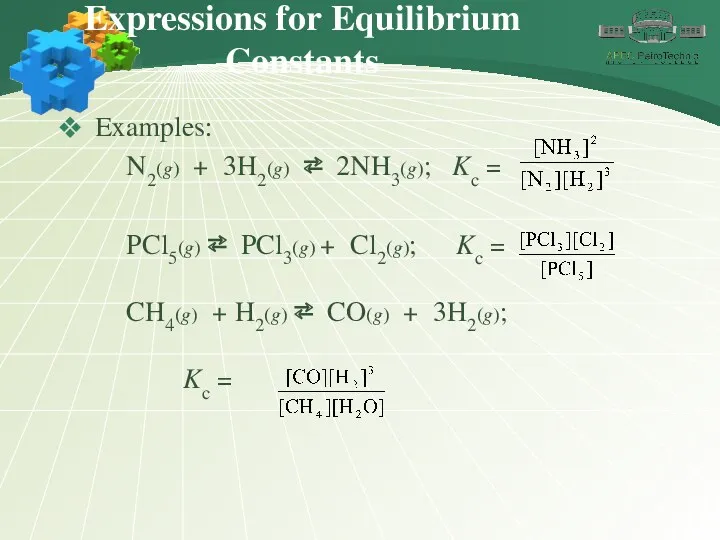
- 8. Expressions for Equilibrium Constants Examples: N2(g) + 3H2(g) ⇄ 2NH3(g); Kc = PCl5(g) ⇄ PCl3(g) +
- 9. Calculating Equilibrium Constant Example-1: 1 mole of H2 gas and 1 mole of I2 vapor are
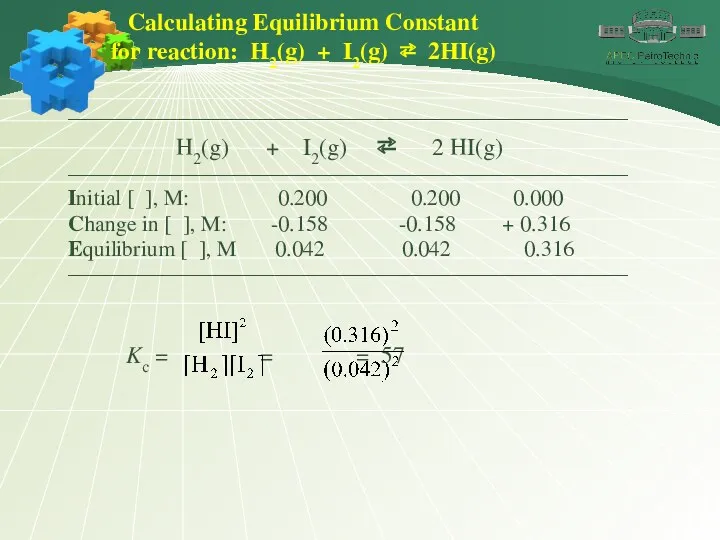
- 10. Calculating Equilibrium Constant for reaction: H2(g) + I2(g) ⇄ 2HI(g) ———————————————————————————— H2(g) + I2(g) ⇄ 2
- 11. Calculating Equilibrium Constant Example-2: 0.500 mole of HI is introduced into a 1.00 liter sealed flask
- 12. Calculating Equilibrium Constant The reaction: H2(g) + I2(g) ⇄ 2HI(g), proceeds from right to left. ————————————————————————————
- 13. Expression and Value of Equilibrium Constant for a Reaction The expression for K depends on the
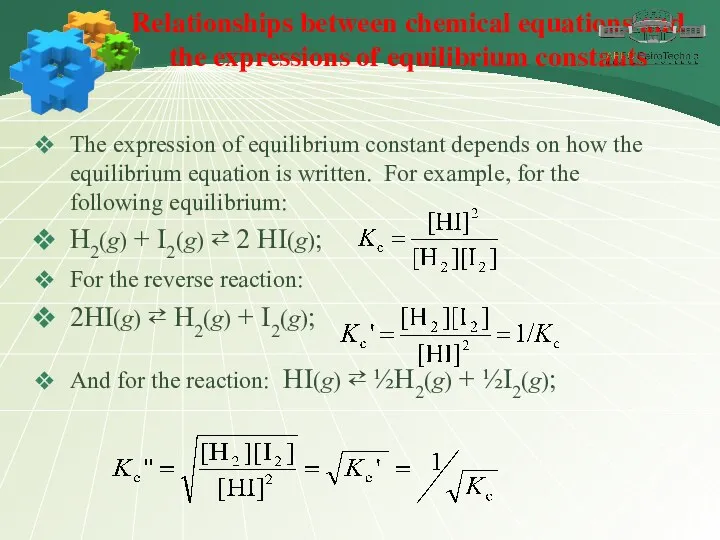
- 14. Relationships between chemical equations and the expressions of equilibrium constants The expression of equilibrium constant depends
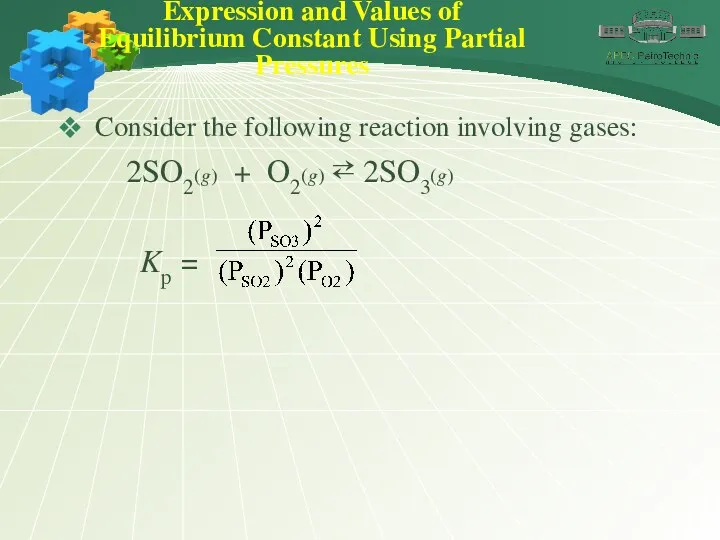
- 15. Expression and Values of Equilibrium Constant Using Partial Pressures Consider the following reaction involving gases: 2SO2(g)
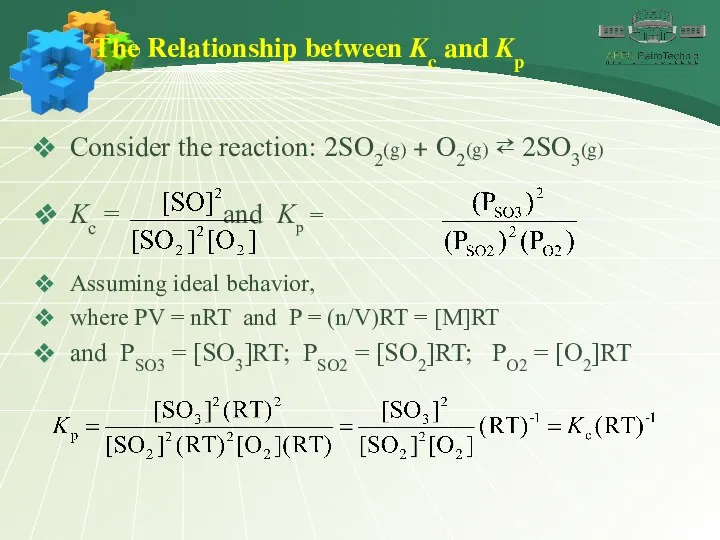
- 16. The Relationship between Kc and Kp Consider the reaction: 2SO2(g) + O2(g) ⇄ 2SO3(g) Kc =
- 17. Relationship between Kc and Kp For reaction: PCl5(g) → PCl3(g) + Cl2(g);
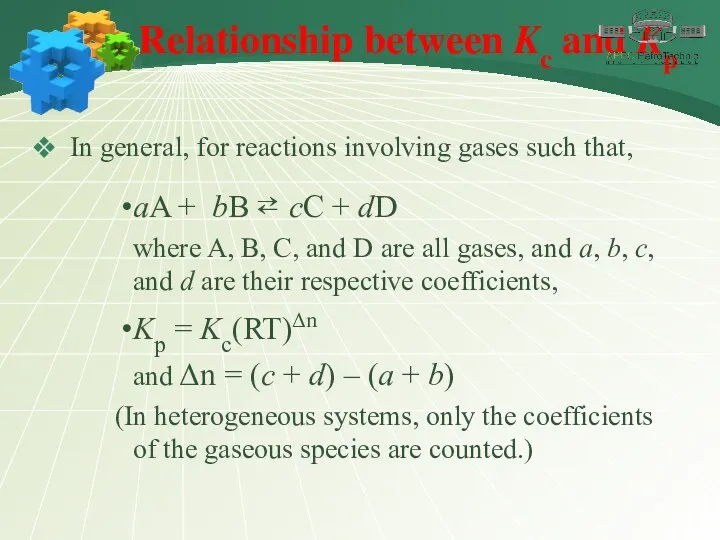
- 18. Relationship between Kc and Kp In general, for reactions involving gases such that, aA + bB
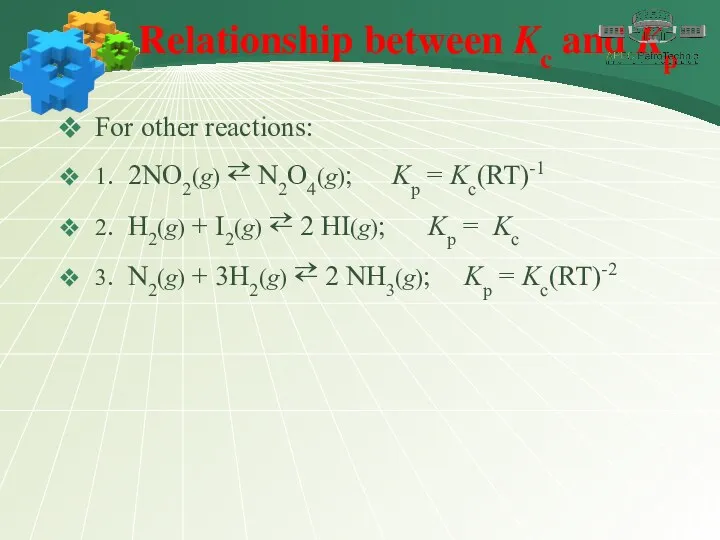
- 19. Relationship between Kc and Kp For other reactions: 1. 2NO2(g) ⇄ N2O4(g); Kp = Kc(RT)-1 2.
- 20. Homogeneous & Heterogeneous Equilibria Homogeneous equilibria: CH4(g) + H2O(g) ⇄ CO(g) + 3H2(g); CO(g) + H2O(g)
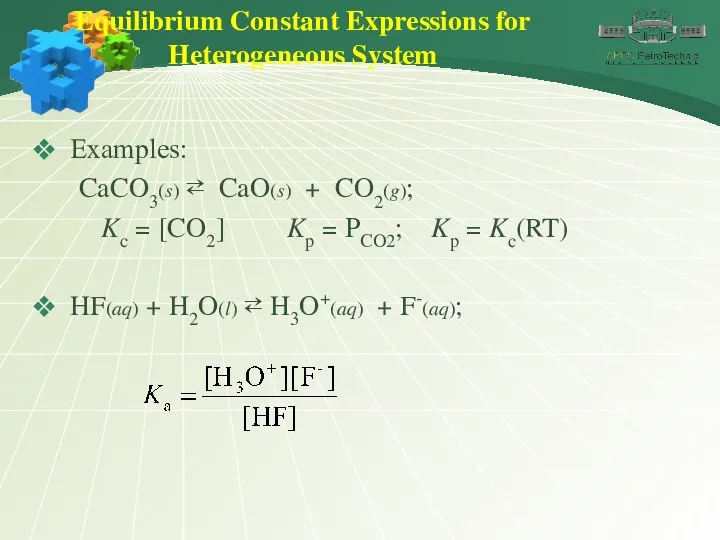
- 21. Equilibrium Constant Expressions for Heterogeneous System Examples: CaCO3(s) ⇄ CaO(s) + CO2(g); Kc = [CO2] Kp
- 22. Solubility Eqilibrium PbCl2(s) ⇄ Pb2+(aq) + 2Cl-(aq); Ksp = [Pb2+][Cl-]2 (Ksp is called solubility product)
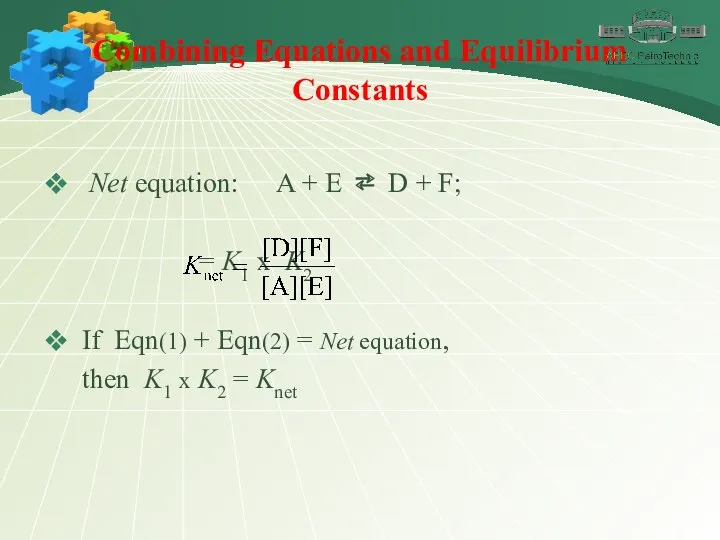
- 23. Combining Equations and Equilibrium Constants when two or more equations are added to yield a net
- 24. Combining Equations and Equilibrium Constants Net equation: A + E ⇄ D + F; = K1
- 25. Equilibrium Exercise #1 A flask is charged with 2.00 atm of nitrogen dioxide and 1.00 atm
- 26. Equilibrium Exercise #2a Methanol is produced according to the following equation: CO(g) + 2H2(g) ⇄ CH3OH(g)
- 27. Equilibrium Exercise #2a Methanol is produced according to the following equation: CO(g) + 2H2(g) ⇄ CH3OH(g)
- 28. Applications of Equilibrium Constant For any system or reaction: Knowing the equilibrium constant, we can predict
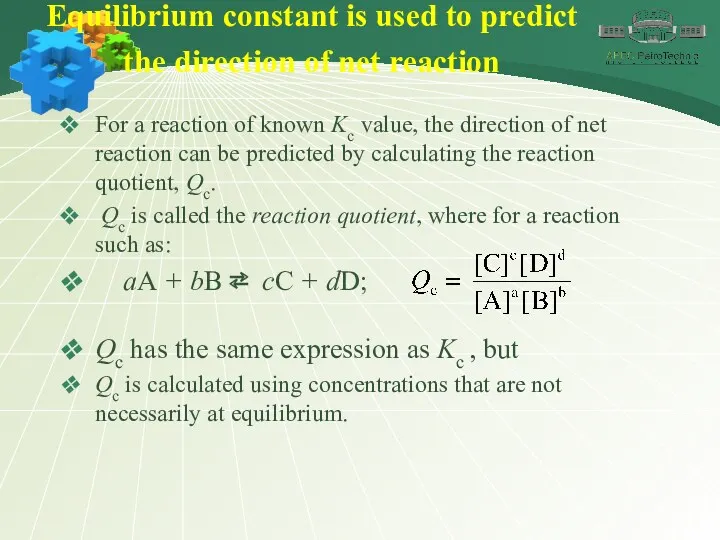
- 29. Equilibrium constant is used to predict the direction of net reaction For a reaction of known
- 30. What does the reaction quotient tell us? If Qc = Kc, ? the reaction is at
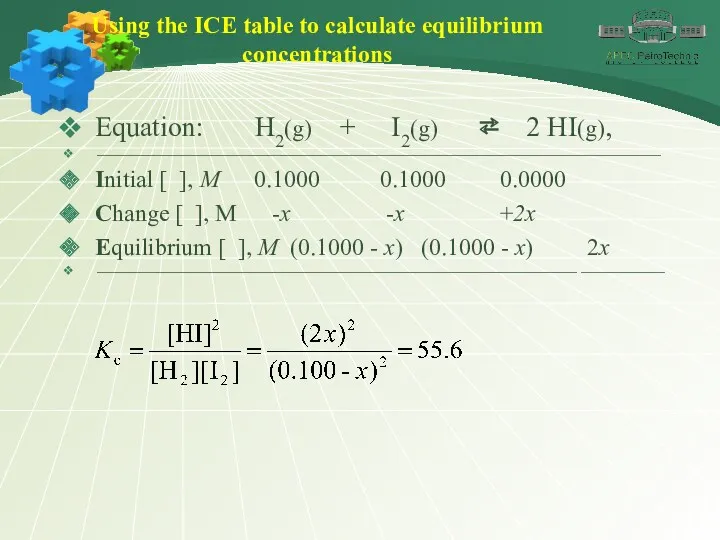
- 31. Using the ICE table to calculate equilibrium concentrations Equation: H2(g) + I2(g) ⇄ 2 HI(g), ⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯
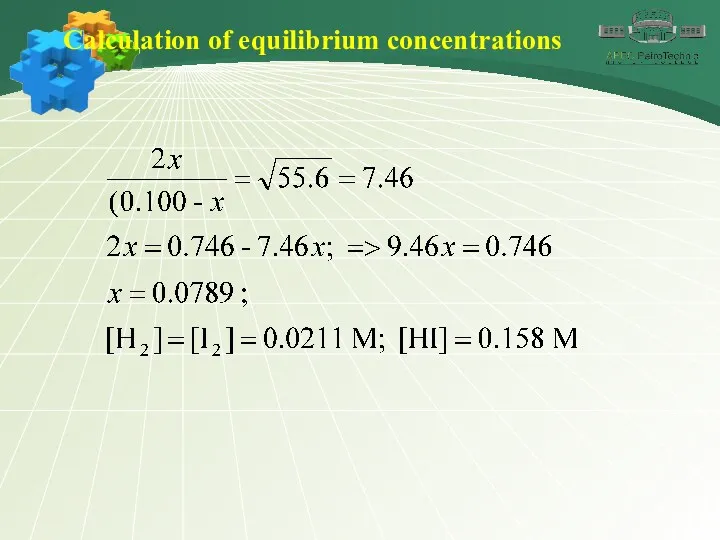
- 32. Calculation of equilibrium concentrations
- 33. Equilibrium Exercise #6 For the reaction: 2 NO2(g) ⇄ N2O4(g); Kp = 1.27 at 353 K.
- 34. Le Châtelier’s Principle The Le Châtelier's principle states that: when factors that influence an equilibrium are
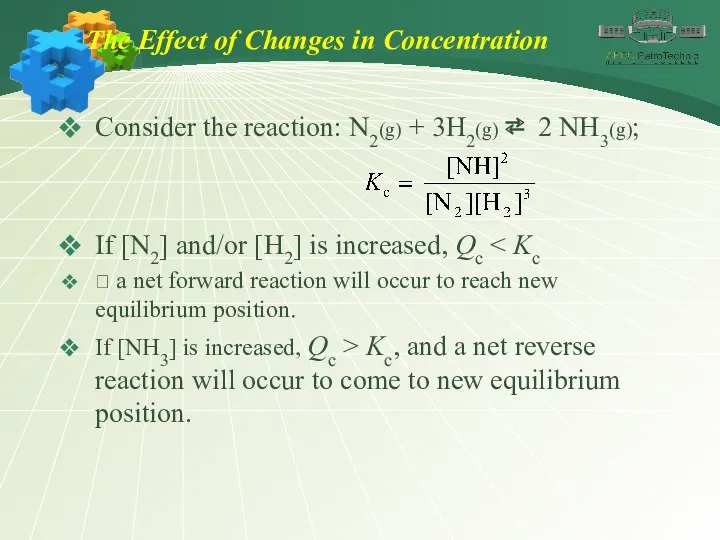
- 35. The Effect of Changes in Concentration Consider the reaction: N2(g) + 3H2(g) ⇄ 2 NH3(g); If
- 36. Reactions that shift right when pressure increases and shift left when pressure decreases Consider the reaction:
- 37. Reaction that shifts left when pressure increases, but shifts right when pressure decreases Consider the reaction:
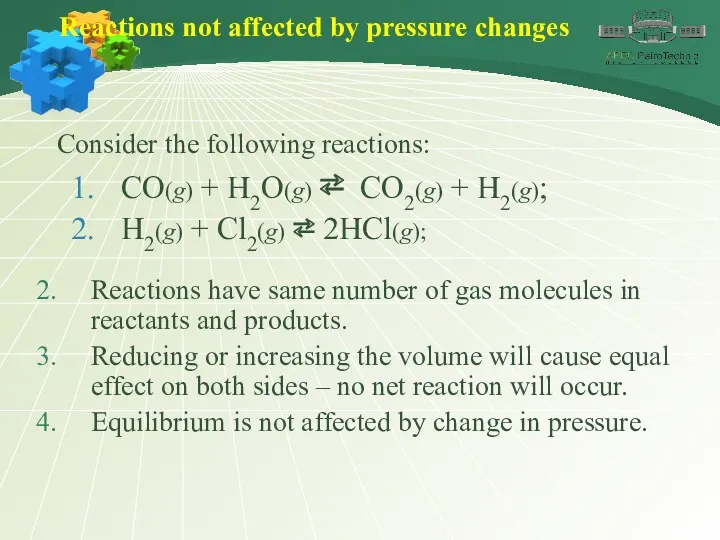
- 38. Reactions not affected by pressure changes Consider the following reactions: CO(g) + H2O(g) ⇄ CO2(g) +
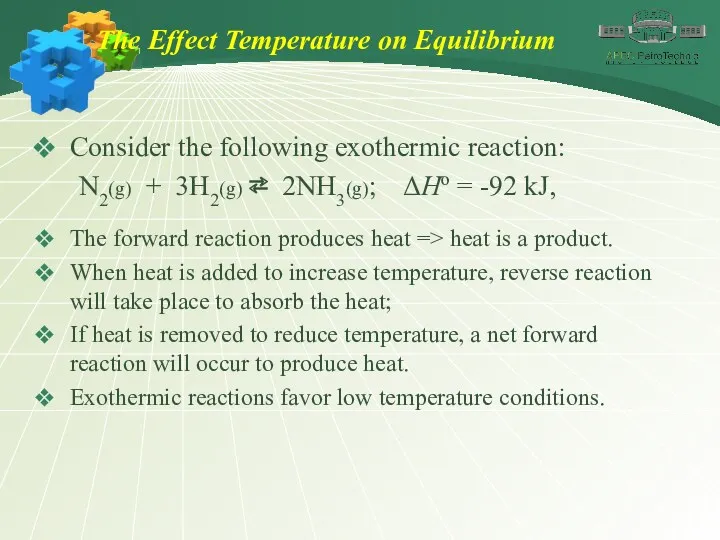
- 39. The Effect Temperature on Equilibrium Consider the following exothermic reaction: N2(g) + 3H2(g) ⇄ 2NH3(g); ΔHo
- 40. Equilibrium Exercise #8 Determine whether the following reactions favor high or low pressures? 2SO2(g) + O2(g)
- 41. Equilibrium Exercise #9 Determine whether the following reactions favors high or low temperature? 2SO2(g) + O2(g)
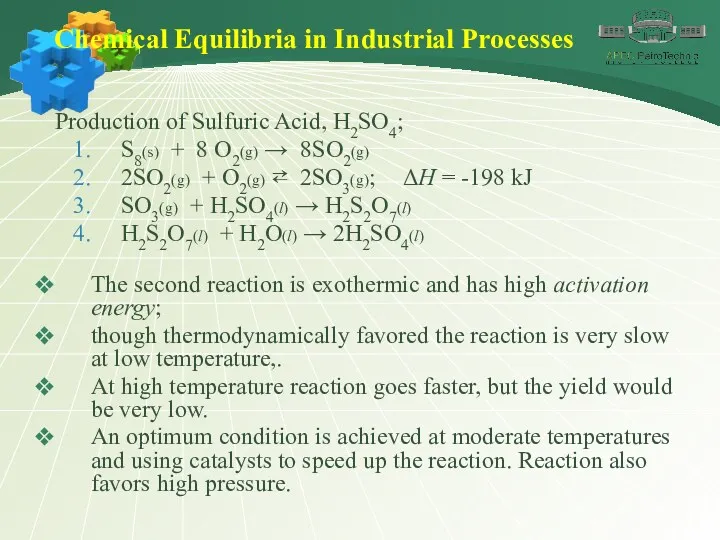
- 42. Chemical Equilibria in Industrial Processes Production of Sulfuric Acid, H2SO4; S8(s) + 8 O2(g) → 8SO2(g)
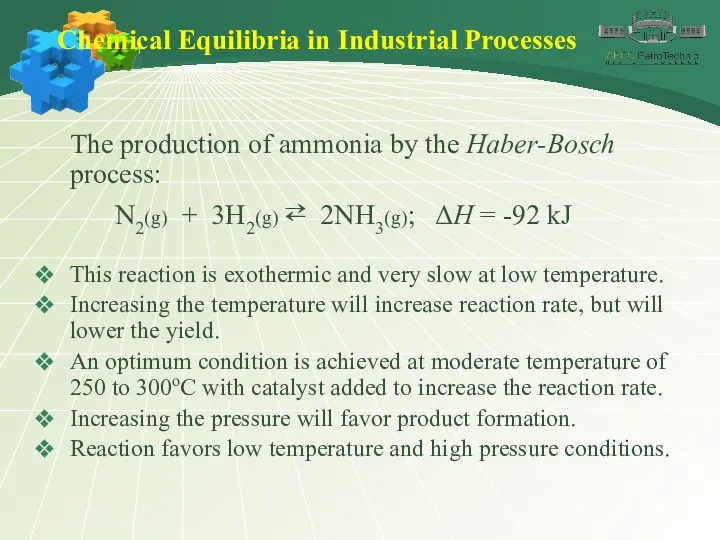
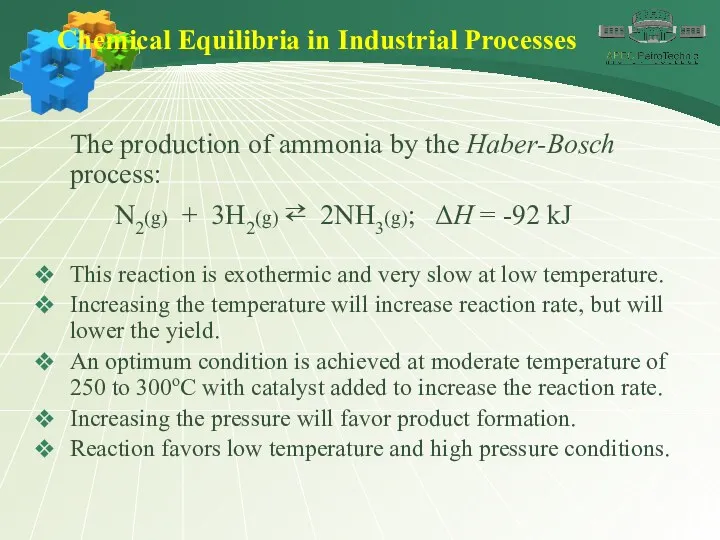
- 43. Chemical Equilibria in Industrial Processes The production of ammonia by the Haber-Bosch process: N2(g) + 3H2(g)
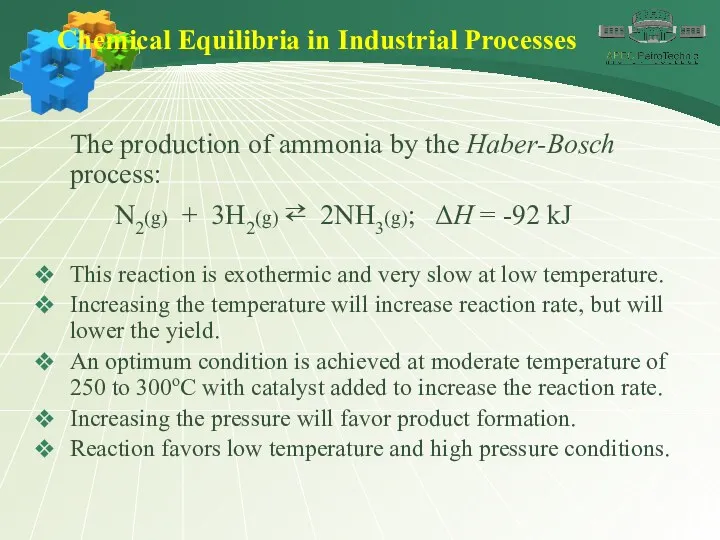
- 44. Chemical Equilibria in Industrial Processes The production of ammonia by the Haber-Bosch process: N2(g) + 3H2(g)
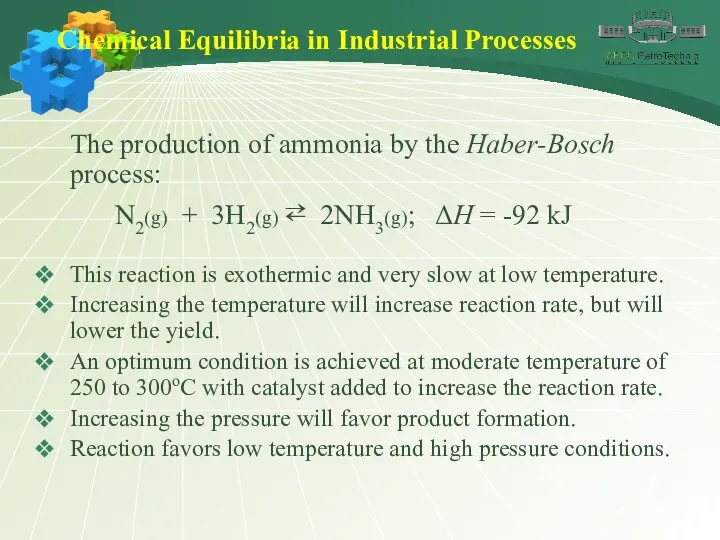
- 45. Chemical Equilibria in Industrial Processes The production of ammonia by the Haber-Bosch process: N2(g) + 3H2(g)
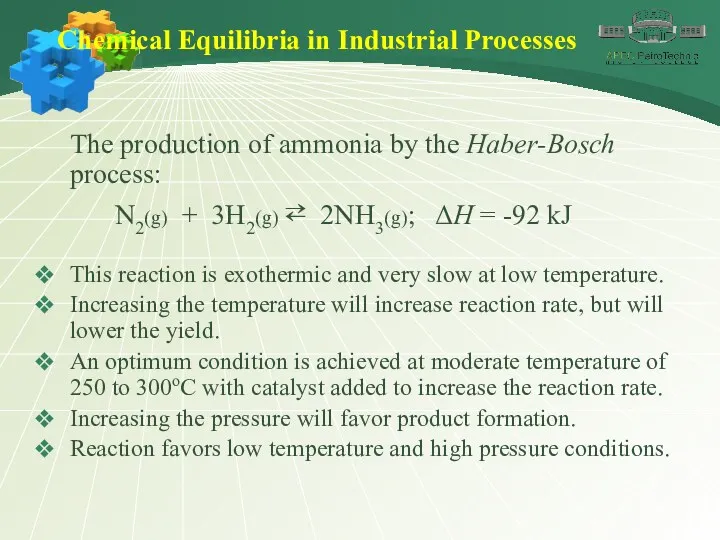
- 46. Chemical Equilibria in Industrial Processes The production of ammonia by the Haber-Bosch process: N2(g) + 3H2(g)
- 47. Chemical Equilibria in Industrial Processes The production of ammonia by the Haber-Bosch process: N2(g) + 3H2(g)
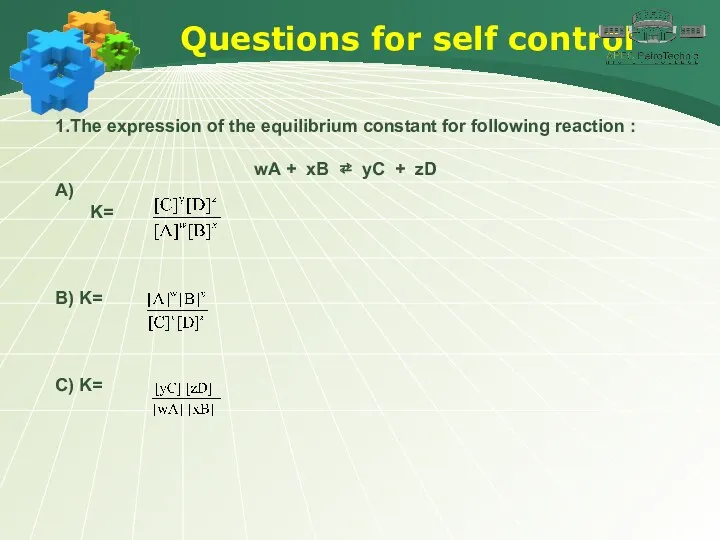
- 48. Questions for self control 1.The expression of the equilibrium constant for following reaction : wA +
- 49. 2)1.000 mole of H2 gas and 1.000 mole of I2 vapor are introduced into a 5.00-liter
- 50. 3.On the base Le-Shatelie principle predict the reaction direction of following reaction : 2SO2(g) + O2(g)
- 51. Literature 1.Basic literature : 1. Jenkins, Chemistry, ISBN 978-0-17-628930-0 2. Alberta Learning, Chemistry data booklet 2010,
- 52. 2.Additional literature : 1.Б.А.Мансуров «Химия» 10-11 кл., Атамура 2015 г 2.Б.Мансуров., Н.Торшина «Методика преподавания органической химии»
- 54. Скачать презентацию









![Solubility Eqilibrium PbCl2(s) ⇄ Pb2+(aq) + 2Cl-(aq); Ksp = [Pb2+][Cl-]2 (Ksp is called solubility product)](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/604317/slide-21.jpg)
















 Буферные растворы. Граф структуры. Теория электрической диссоциации. Химическое равновесие
Буферные растворы. Граф структуры. Теория электрической диссоциации. Химическое равновесие Оксиды. Классификация. Номенклатура. Свойства оксидов. Получение. Применение
Оксиды. Классификация. Номенклатура. Свойства оксидов. Получение. Применение Этанол (эти́ловый спирт)
Этанол (эти́ловый спирт) Галогены. Свойства
Галогены. Свойства Периодическая таблица Д.И. Менделеева. Своя игра
Периодическая таблица Д.И. Менделеева. Своя игра Массовая доля вещества в растворе
Массовая доля вещества в растворе Общая электронная теория восстановления и окисления металлов
Общая электронная теория восстановления и окисления металлов Витамины молока и молочных продуктов. Жирорастворимые витамины
Витамины молока и молочных продуктов. Жирорастворимые витамины Электрохимическая коррозия
Электрохимическая коррозия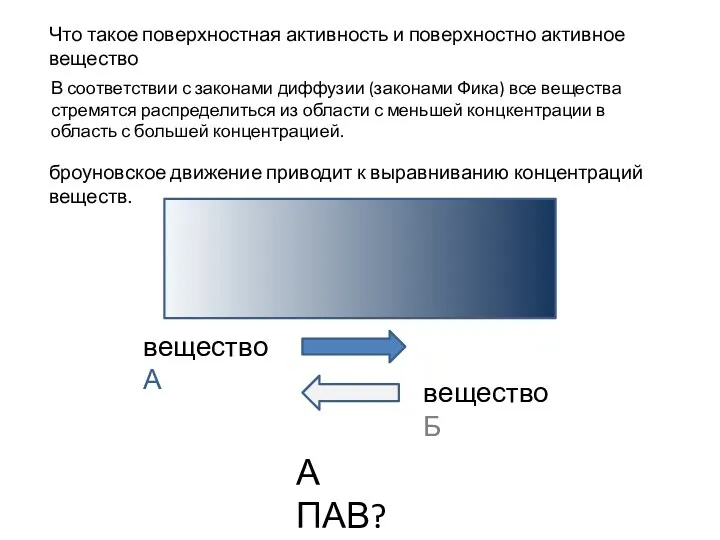 Поверхностная активность и поверхностно активное вещество
Поверхностная активность и поверхностно активное вещество Поняття про родини хімічних елементів: лужні метали, галогени, інертні елементи. Урок хімії у 8 класі
Поняття про родини хімічних елементів: лужні метали, галогени, інертні елементи. Урок хімії у 8 класі Р-элементы IV Группы Периодической Системы
Р-элементы IV Группы Периодической Системы Механизмы органических реакций
Механизмы органических реакций Химическое равновесие. Смещение химического равновесия
Химическое равновесие. Смещение химического равновесия Электронные конфигурации атомов
Электронные конфигурации атомов Химия и продукты питания
Химия и продукты питания Химическая связь и ее типы. (11 класс)
Химическая связь и ее типы. (11 класс) Аналитическая химия
Аналитическая химия Синтетические каучуки: хлоропреновый каучук
Синтетические каучуки: хлоропреновый каучук Многоатомные спирты
Многоатомные спирты Мінеральні добрива
Мінеральні добрива Основные сведения о строении атома
Основные сведения о строении атома Природные источники углеводородов
Природные источники углеводородов Колебания кристаллической решетки и ее тепловые свойства. Тепловые свойства
Колебания кристаллической решетки и ее тепловые свойства. Тепловые свойства Свойства кислот и оснований в свете теории электролитической диссоциации
Свойства кислот и оснований в свете теории электролитической диссоциации АТФ Аденозинтрифосфат
АТФ Аденозинтрифосфат c0198e3edf1db804a5527004a7864ed1
c0198e3edf1db804a5527004a7864ed1 Высокомолекулярные соединения. Общий курс
Высокомолекулярные соединения. Общий курс