Содержание
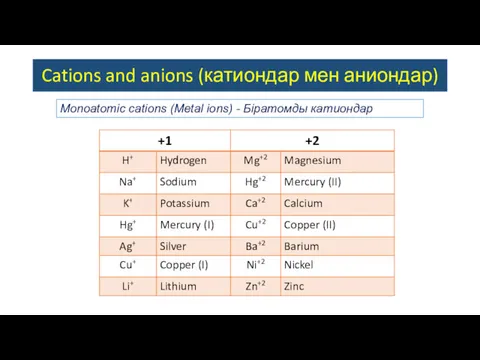
- 2. Cations and anions (катиондар мен аниондар) Monoatomic сations (Metal ions) - Біратомды катиондар
- 3. Monoatomic сations (Metal ions) - Біратомды катиондар Polyatomic Cations – Көпатомды катиондар Cations and anions (катиондар
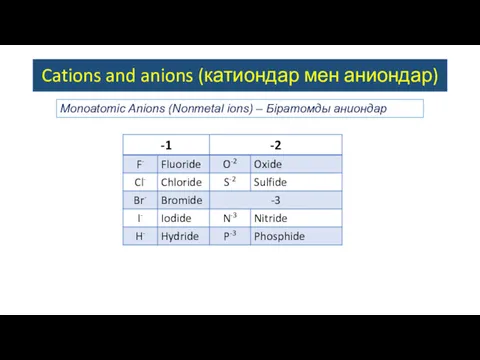
- 4. Monoatomic Anions (Nonmetal ions) – Біратомды аниондар Cations and anions (катиондар мен аниондар)
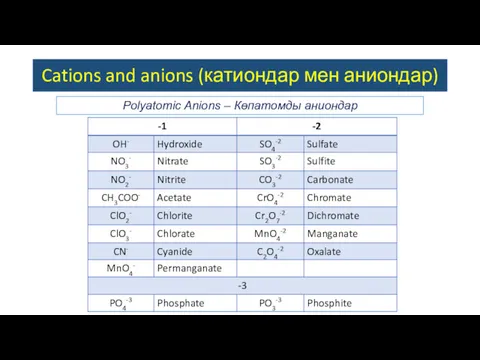
- 5. Polyatomic Anions – Көпатомды аниондар Cations and anions (катиондар мен аниондар)
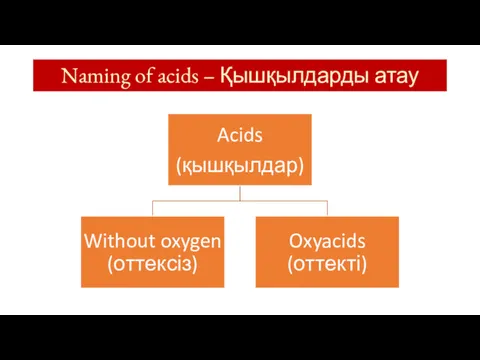
- 6. Naming of acids – Қышқылдарды атау
- 7. Naming of acids – Қышқылдарды атау HCl : Hydrochloric acid HI : Hydroiodic acid H2S :
- 8. Naming of bases – Негіздерді атау KOH : Potassium hydroxide NaOH : Sodium hydroxide Mg(OH)2 :
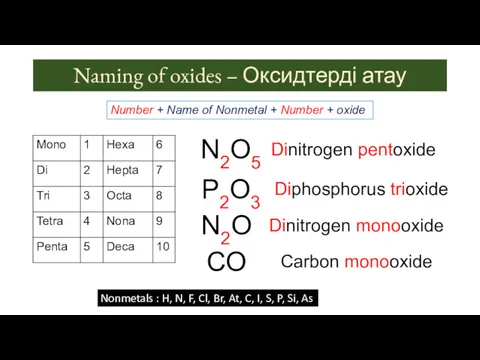
- 9. Naming of oxides – Оксидтерді атау Number + Name of Nonmetal + Number + oxide Nonmetals
- 10. Naming of oxides – Оксидтерді атау Name of metal (charge)+ oxide Metals : Li, Na, K,
- 12. Скачать презентацию




 Е-числа в школьном буфете
Е-числа в школьном буфете Общие проблемы определения низких концентраций
Общие проблемы определения низких концентраций Особенности строения твердых тел
Особенности строения твердых тел Контрольная работа по дисциплине Физическая химия. Раздел: Электрохимия
Контрольная работа по дисциплине Физическая химия. Раздел: Электрохимия Химия элементов
Химия элементов Элементы VI группы главной подгруппы
Элементы VI группы главной подгруппы Биологически важные пяти- и шестичленные гетероциклы с одним и двумя гетероатомами
Биологически важные пяти- и шестичленные гетероциклы с одним и двумя гетероатомами Законы химии
Законы химии Олигомеризация олефинов
Олигомеризация олефинов Комбинированные задачи. 11 класс
Комбинированные задачи. 11 класс Чистые вещества и смеси
Чистые вещества и смеси Полимеры. Структура и свойства
Полимеры. Структура и свойства Водород. Химические свойства
Водород. Химические свойства Неметали. Фізичні та хімічні властивості. Явище адсорбції. Сполуки неметалічних елементів з Гідрогеном
Неметали. Фізичні та хімічні властивості. Явище адсорбції. Сполуки неметалічних елементів з Гідрогеном Кислоты, основания, соли в свете ТЭД
Кислоты, основания, соли в свете ТЭД Шыны, әйнек
Шыны, әйнек Серебро. История
Серебро. История Химическая кинетика
Химическая кинетика Поверхностные явления. Типы поверхностных явлений
Поверхностные явления. Типы поверхностных явлений Важнейшие классы бинарных соединений - оксиды и летучие водородные соединения
Важнейшие классы бинарных соединений - оксиды и летучие водородные соединения Способы выражения состава раствора
Способы выражения состава раствора альдегиды, свойства, получение
альдегиды, свойства, получение Coordination compounds
Coordination compounds Растворы. (Лекция 7)
Растворы. (Лекция 7) Сера. Нахождение в природе. Химические свойства серы
Сера. Нахождение в природе. Химические свойства серы Гравиметрический метод анализа
Гравиметрический метод анализа Оксид цинка
Оксид цинка Анализ качества лекарственных веществ, определяемых методом комплексонометрии
Анализ качества лекарственных веществ, определяемых методом комплексонометрии