Содержание
- 2. 7.1 Introduction Presence of counterions (= gegenions) Influence of counterions Solvation effect more complex than free
- 3. 7.1 Introduction
- 4. TABLE 7.1. Commercially Important Polymers Prepared by Ionic Polymerization Polymer or Copolymer Cationica Polyisobutylene and polybuteneb
- 5. 7.2.1 Cationic initiators 7.2.2 Mechanism, kinetics, and reactivity in cationic polymerization 7.2.3 Stereochemistry of cationic polymerization
- 6. 7.2.1 Cationic Initiators The propagating species : carbocation Coinitiator
- 7. (7.5) (7.6) (7.7) (7.8) Other initiators 7.2.1 Cationic Initiators
- 8. Other initiators 7.2.1 Cationic Initiators
- 9. 7.2.2 Mechanism, Kinetics, and Reactivity in Cationic Polymerization Carbocationic Initiation. addition of the electrophilic species –
- 10. 7.2.2 Mechanism, Kinetics, and Reactivity in Cationic Polymerization Carbocationic Initiation.
- 11. B. Propagation Step 7.2.2 Mechanism, Kinetics, and Reactivity in Cationic Polymerization
- 12. C. Influences polymerization rate
- 13. D. Chain transfer reaction
- 14. D. Chain transfer reaction
- 15. E. Termination reaction
- 16. F. Proton trap
- 17. G. Telechelic Polymer
- 18. H. Pseudocationic Polymerization
- 19. I. To prepare living polymers under cationic conditions.
- 20. I. To prepare living polymers under cationic conditions.
- 21. J. Kinetics
- 23. Substituting for in , one obtains In the absence of any chain transfer, (the kinetic chain
- 24. K. Difference between free radical and cationic processes.
- 25. L. Nonconjugation diene – Cationic cyclopolymerization 7.2.2 Mechanism, Kinetics, and Reactivity in Cationic Polymerization
- 26. Cationic Polymerization lead to stereoregular structures. ex) vinyl ether α - methylstyrene Vinyl ether observation resulting
- 27. EX) t-butyl vinyl ether forms isotactic polymer in nonpolar solvents. forms mainly syndiotactic polymer in polar
- 28. In polar solvents both ions 1) be strongly solvated 2) the chain end – exist as
- 29. (7.29) (7.30) Models proposed for vinyl ether polymerization
- 31. 7.2.4 Cationic Copolymerization A. Copolymerization equation - the situation is complication by counterion effects. B. Reactivity
- 32. TABLE 7.3. Representative Cationic Reactivity Rations (r)a Monomer 1 Monomer 2 Coinitiatorb Solventb Temperature (oC) r1
- 33. 7.2.5 Isomerization in Cationic Polymerization (7.34) (7.35)
- 34. 7.3 Anionic Polymerization 7.3.1 Anionic initiators 7.3.2 Mechanism, kinetics, and reactivity in anionic polymerization 7.3.3 Stereochemistry
- 35. (7.36) Propagating chain - carbanion Examples – nitro, cyano, carboxyl, vinyl, and phenyl. Monomers having substituent
- 36. The strength of the base necessary to initiate polymerization depends in large measure on monomer structure
- 37. Two basic types that react by addition of a negative ion that undergo electron transfer. ①
- 38. 7.3.1 Anionic Initiators
- 39. 7.3.2 Mechanism, kinetics, and reactivity in anionic polymerization A. Mechanism을 변화시킬 수 있는 요인 a. solvent
- 40. b. Type of cation (counterion) c. Temperature B. The rate of initiation - initiator 와 monomer의
- 41. D. Kinetic 7.3.2 Mechanism, kinetics, and reactivity in anionic polymerization Because the second step is slow
- 42. Substituting in Rp we obtain The average kinetic chain length, is expressed as Assuming a steady
- 43. E. Other types of transfer reactions 7.3.2 Mechanism, kinetics, and reactivity in anionic polymerization
- 44. 7.3.2 Mechanism, kinetics, and reactivity in anionic polymerization
- 45. 7.3.2 Mechanism, kinetics, and reactivity in anionic polymerization G. Important factor in propagation rate. a. Association
- 46. 7.3.2 Mechanism, kinetics, and reactivity in anionic polymerization G. Important factor in propagation rate.
- 47. 7.3.3 Stereochemistry of anionic polymerization A. Stereochemical of nondiene vinyl monomer With soluble anionic initiators (homogeneous
- 48. 7.3.3 Stereochemistry of anionic polymerization A. Stereochemical of nondiene vinyl monomer
- 49. 7.3.3 Stereochemistry of anionic polymerization A. Stereochemical of nondiene vinyl monomer Effect of solvent
- 50. B. Stereochemical of Dienes 7.3.3 Stereochemistry of anionic polymerization catalyst, solvent의 영향 isoprene 1,3-butadiene Li-based initiator/nonpolar
- 51. formation of cis-polyisoprene – lithium’s ability forming a six-membered ring transition state – “lock” the isoprene
- 52. 7.3.4 Anionic Copolymerization ④ contrasts between homogeneous and heterogeneous polymerization systems.
- 54. 7.3.4 Anionic Copolymerization formation of block copolymers by the living polymer method.
- 55. ABA triblock polymers – Greatest commercial success ex) styrene-butadiene-styrene star-block (radial) – much lower melt viscosities,
- 56. 7.4 Group Transfer Polymerization (GTP) (In the 1980s a new method for polymerizing acrylic-type monomers) GTP의
- 58. 7.4 Group Transfer Polymerization (GTP) 두 개의 작용기를 갖는 개시제 사용 사슬의 양끝에서 성장
- 59. 7.4 Group Transfer Polymerization (GTP)
- 60. 7.4 Group Transfer Polymerization (GTP)
- 62. Скачать презентацию



























































 Сапалық талдау. Сапалық аналитикалық реакциялар
Сапалық талдау. Сапалық аналитикалық реакциялар Комплексті қосылыстар және олардың биологиялық маңызы
Комплексті қосылыстар және олардың биологиялық маңызы Обчислення швидкості хімічних реакцій в залежності від концентрації реагуючих речовин і температури
Обчислення швидкості хімічних реакцій в залежності від концентрації реагуючих речовин і температури Масса и формулы. Химия 8 класс
Масса и формулы. Химия 8 класс Алкадиены
Алкадиены O-alkylation catalysts
O-alkylation catalysts Карбон қышқылдары, жіктелуі, сипаттамалары, таралуы
Карбон қышқылдары, жіктелуі, сипаттамалары, таралуы Превращение веществ
Превращение веществ Камень чароит
Камень чароит Непредельные углеводороды ряда этилена. Олефины
Непредельные углеводороды ряда этилена. Олефины Спирт. Спирты в природе. Влияние спирта на человека
Спирт. Спирты в природе. Влияние спирта на человека Алюминий и его соединения
Алюминий и его соединения Қатты әсер ететін уландырғыш заттар
Қатты әсер ететін уландырғыш заттар Классы неорганических веществ. Классификация неорганических веществ
Классы неорганических веществ. Классификация неорганических веществ Теория электролитической диссоциации
Теория электролитической диссоциации Основания. 8 класс
Основания. 8 класс Химическая связь. Виды химической связи
Химическая связь. Виды химической связи Карбонові кислоти. Хімія. 9 клас
Карбонові кислоти. Хімія. 9 клас Алкадиены. Непредельные углеводороды
Алкадиены. Непредельные углеводороды Методы разделения и исследования состава нефти и газа
Методы разделения и исследования состава нефти и газа Карбонильные соединения
Карбонильные соединения Особенности организации обучения химии в рамках компетентностно-ориентированной модели образования
Особенности организации обучения химии в рамках компетентностно-ориентированной модели образования Химическая промышленность. Минеральные удобрения
Химическая промышленность. Минеральные удобрения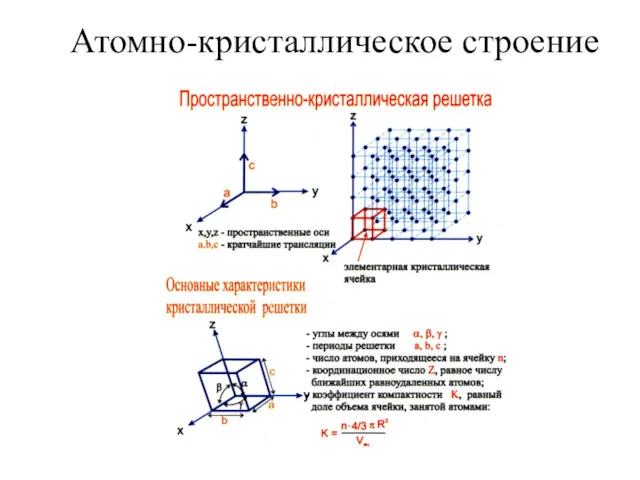 Атомно-кристаллическое строение
Атомно-кристаллическое строение Углеводороды из Башкортостана
Углеводороды из Башкортостана Классификация органических соединений. Углеводороды
Классификация органических соединений. Углеводороды Окислительно-восстановительные реакции. 11 класс
Окислительно-восстановительные реакции. 11 класс Катионы 1, 2 аналитических групп
Катионы 1, 2 аналитических групп