Содержание
- 2. Organic Compounds Containing Oxygen - III Session
- 3. Session Objectives Properties of phenols Reaction of phenols Preparation of ethers Properties and reactions of ethers
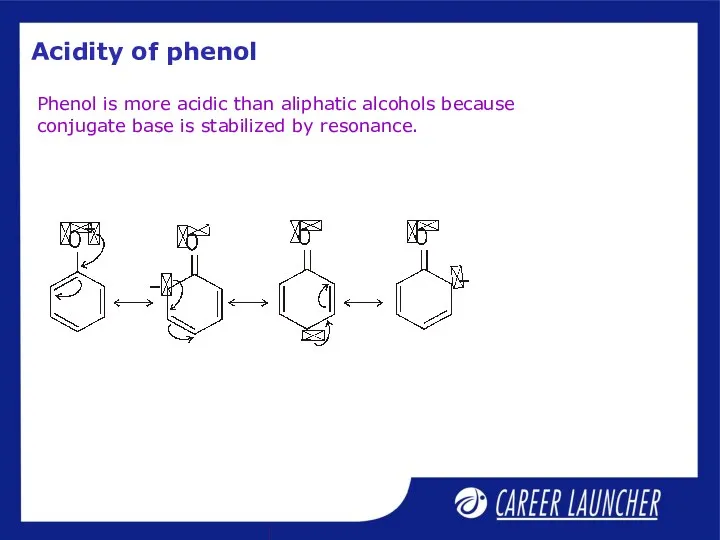
- 4. Acidity of phenol Phenol is more acidic than aliphatic alcohols because conjugate base is stabilized by
- 5. Reactions of phenol Electrophilic aromatic substitution —OH group is ortho, para- directing group and activates the
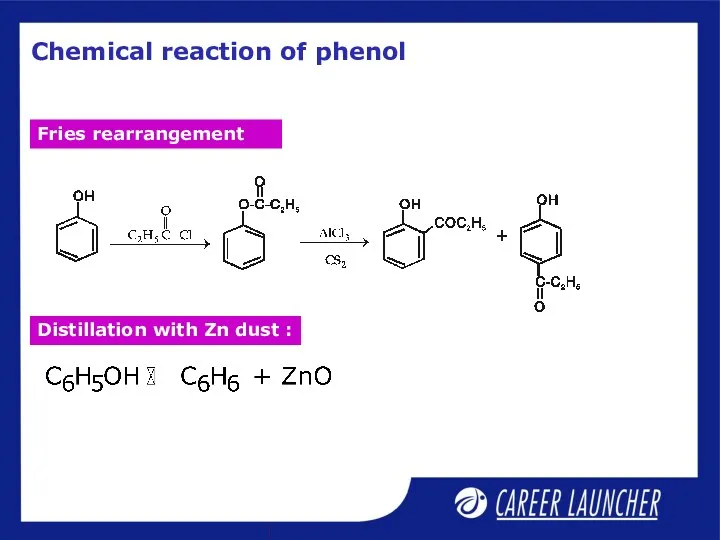
- 6. Chemical reaction of phenol Fries rearrangement Distillation with Zn dust :
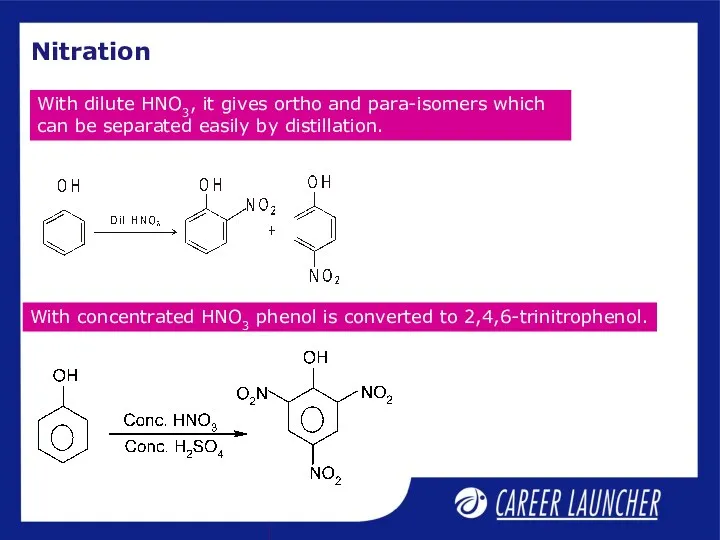
- 7. Nitration With dilute HNO3, it gives ortho and para-isomers which can be separated easily by distillation.
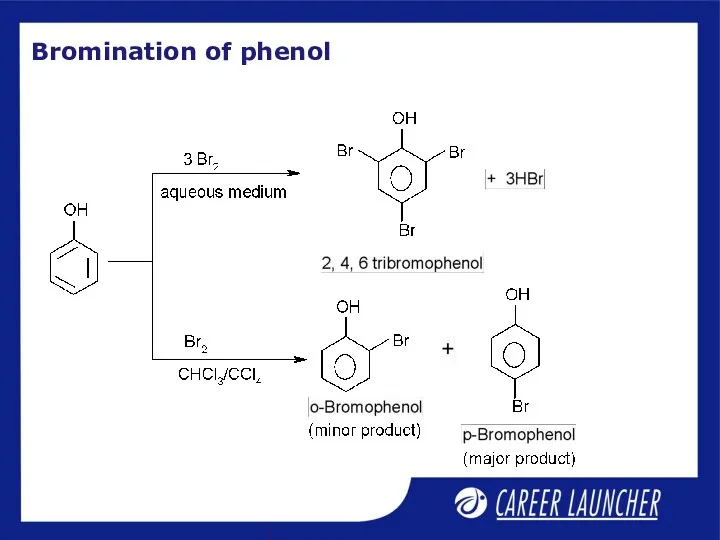
- 8. Bromination of phenol
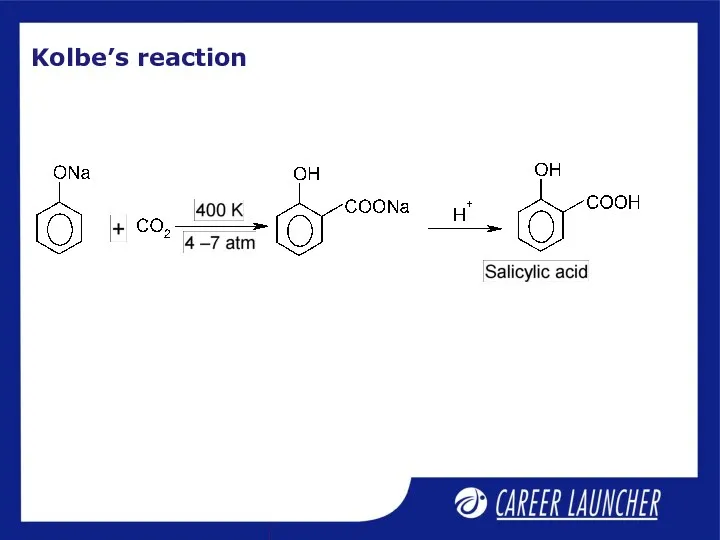
- 9. Kolbe’s reaction
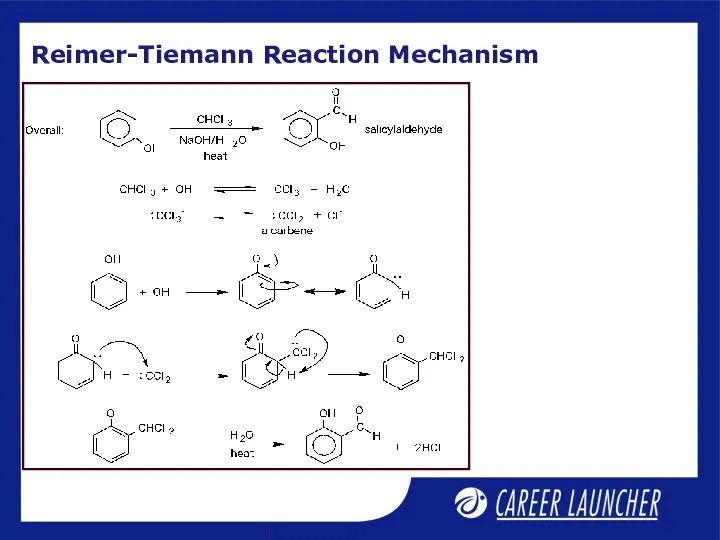
- 10. Reimer-Tiemann Reaction Mechanism
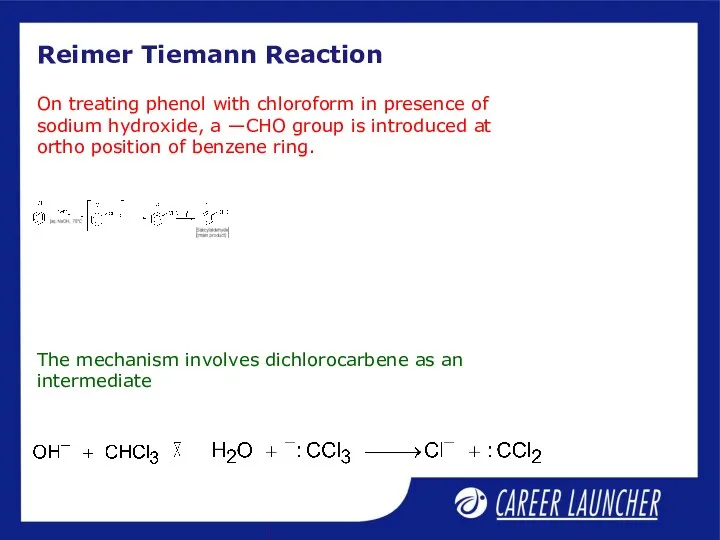
- 11. Reimer Tiemann Reaction The mechanism involves dichlorocarbene as an intermediate On treating phenol with chloroform in
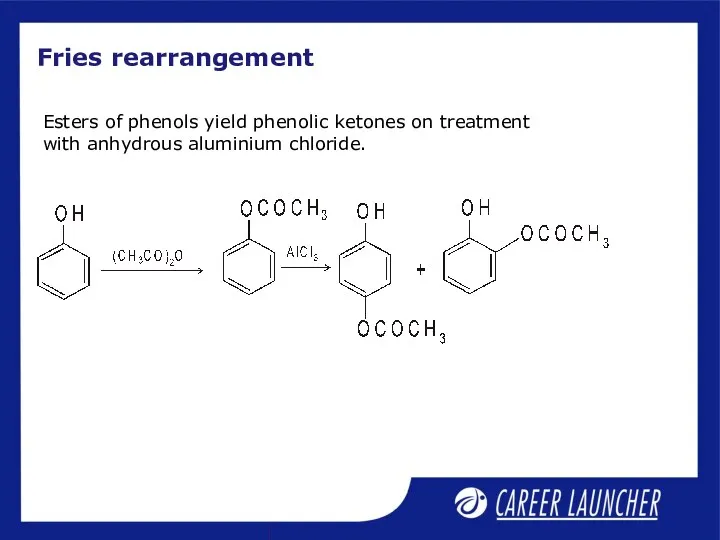
- 12. Fries rearrangement Esters of phenols yield phenolic ketones on treatment with anhydrous aluminium chloride.
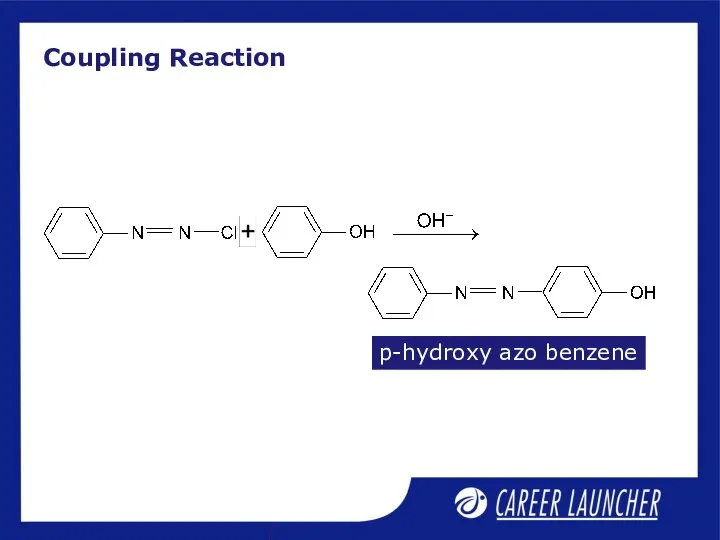
- 13. Coupling Reaction p-hydroxy azo benzene
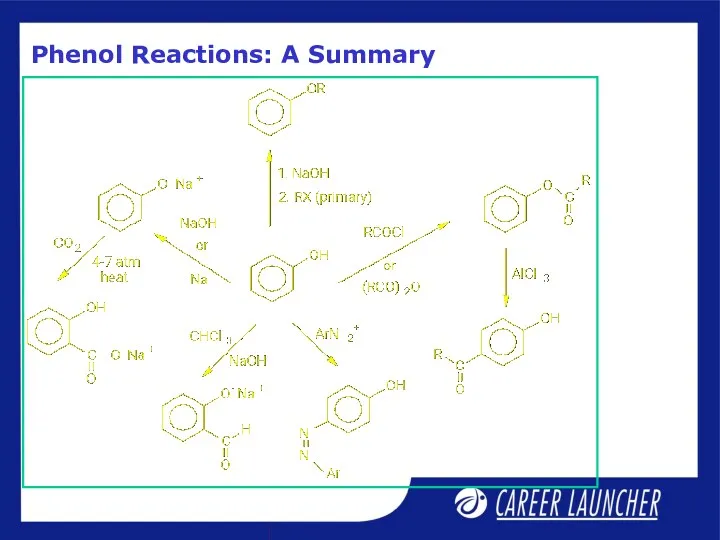
- 14. Phenol Reactions: A Summary
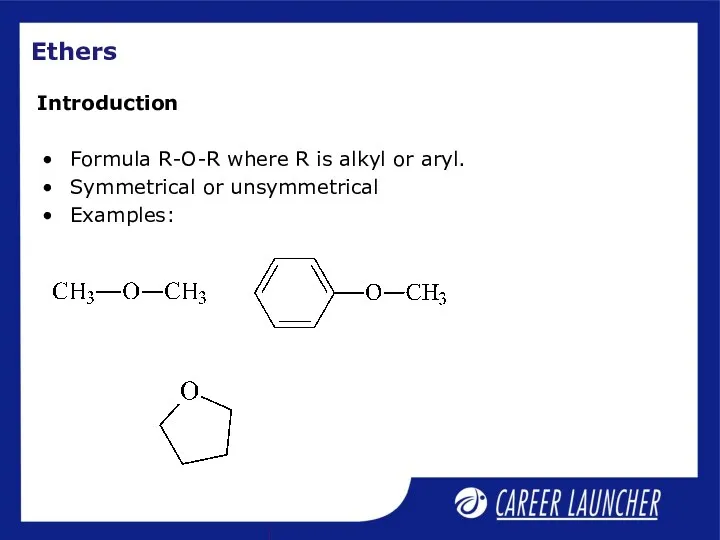
- 15. Ethers Formula R-O-R where R is alkyl or aryl. Symmetrical or unsymmetrical Examples: Introduction
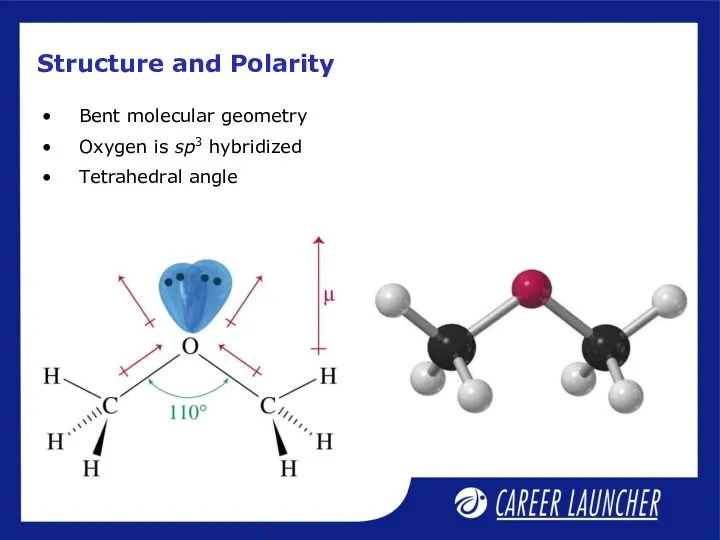
- 16. Structure and Polarity Bent molecular geometry Oxygen is sp3 hybridized Tetrahedral angle
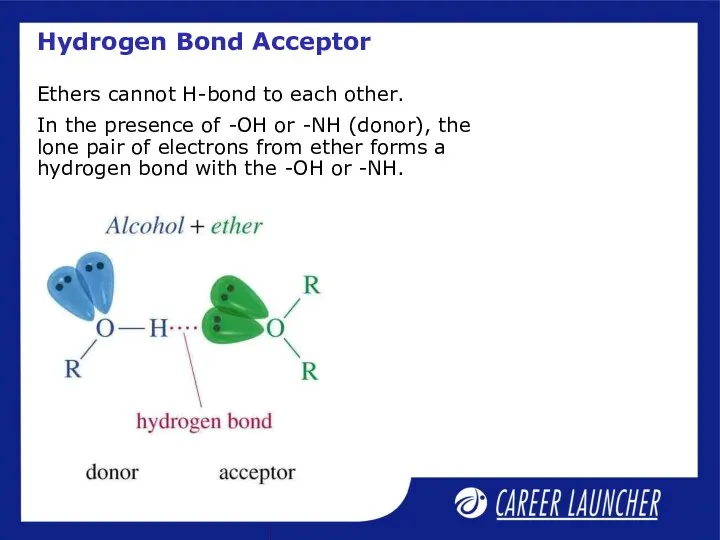
- 17. Hydrogen Bond Acceptor Ethers cannot H-bond to each other. In the presence of -OH or -NH
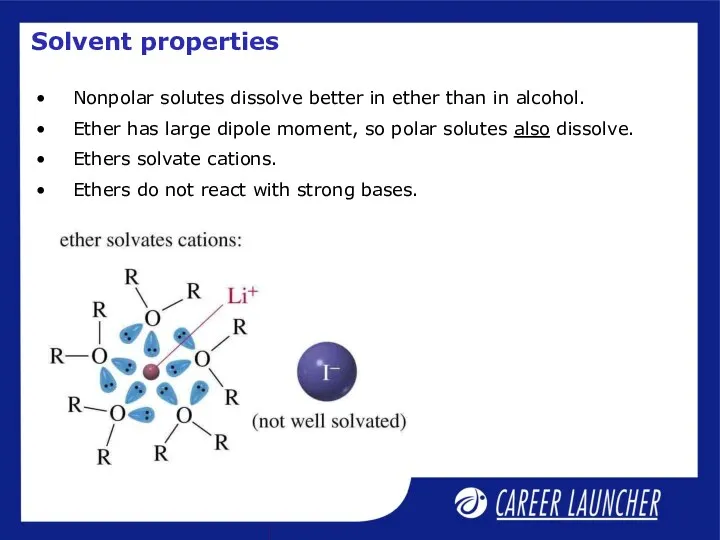
- 18. Solvent properties Nonpolar solutes dissolve better in ether than in alcohol. Ether has large dipole moment,
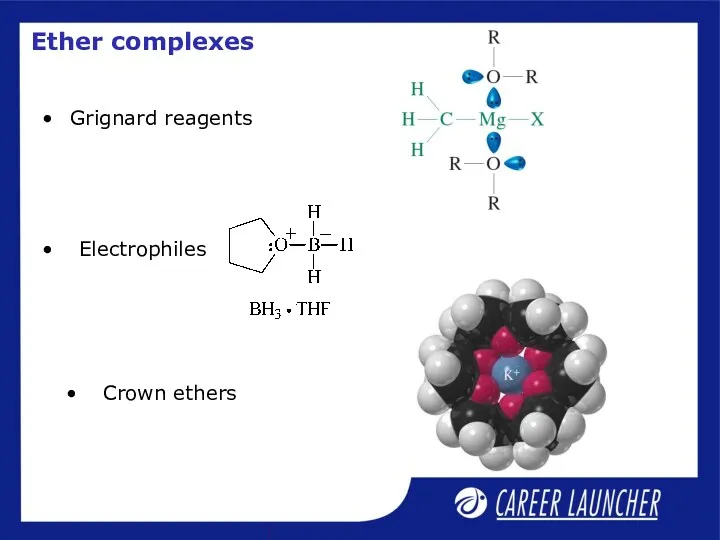
- 19. Grignard reagents Ether complexes Crown ethers Electrophiles
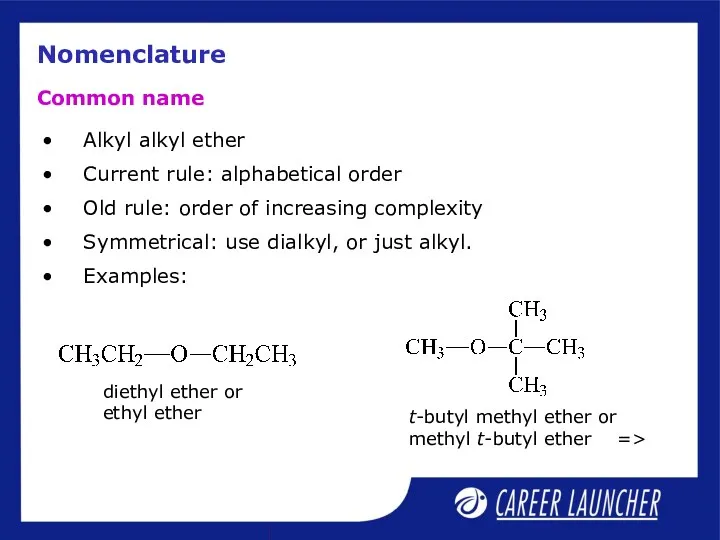
- 20. Nomenclature Alkyl alkyl ether Current rule: alphabetical order Old rule: order of increasing complexity Symmetrical: use
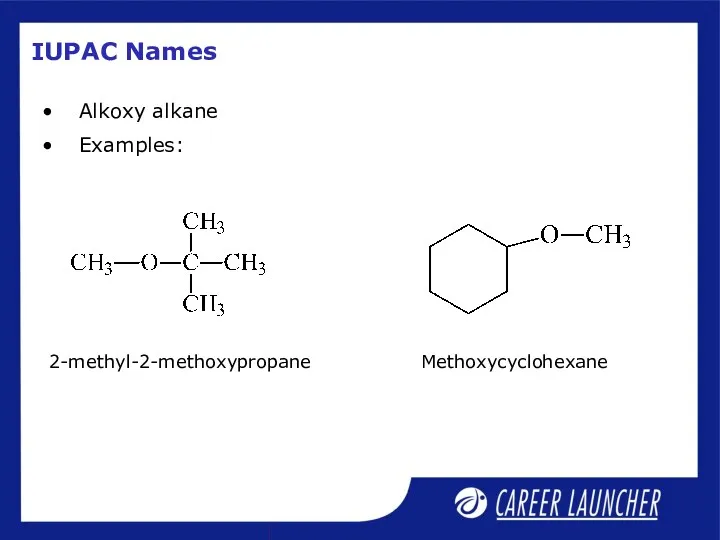
- 21. IUPAC Names Alkoxy alkane Examples:
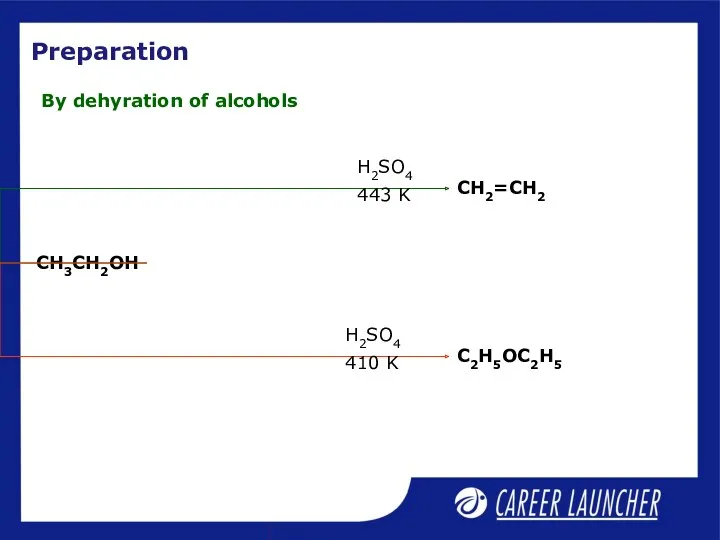
- 22. Preparation By dehyration of alcohols
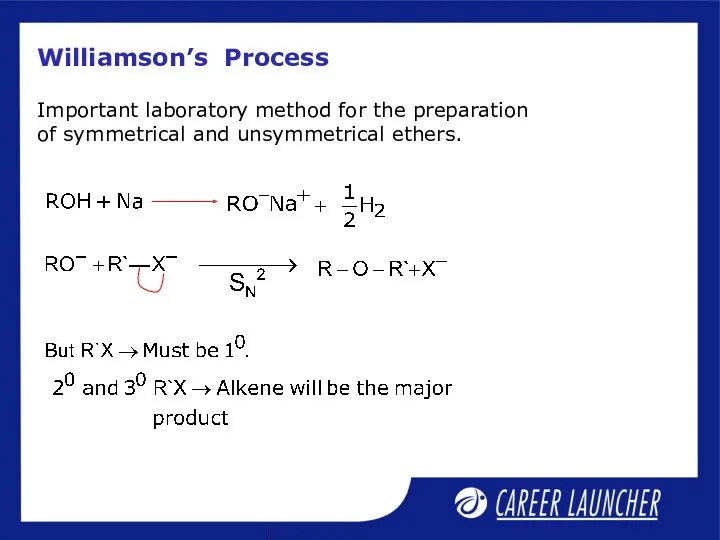
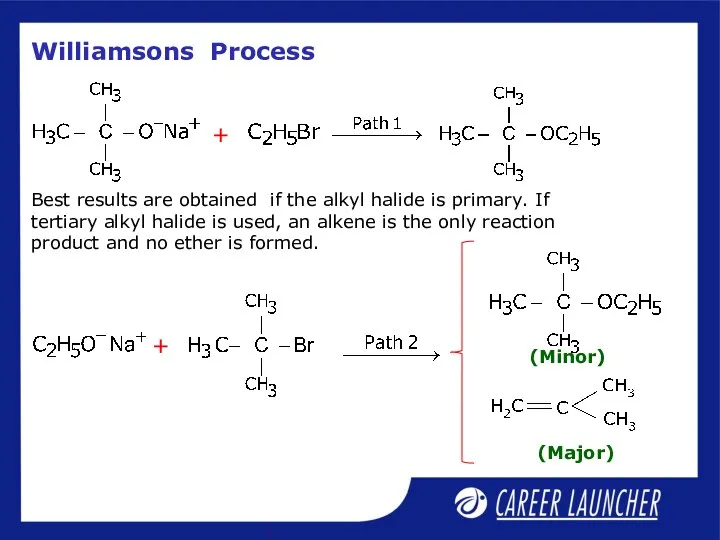
- 23. Williamson’s Process Important laboratory method for the preparation of symmetrical and unsymmetrical ethers.
- 24. Williamsons Process Best results are obtained if the alkyl halide is primary. If tertiary alkyl halide
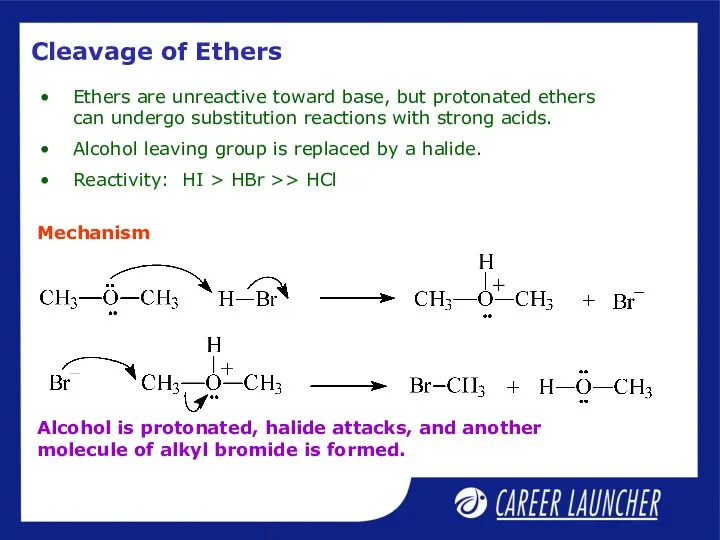
- 25. Cleavage of Ethers Ethers are unreactive toward base, but protonated ethers can undergo substitution reactions with
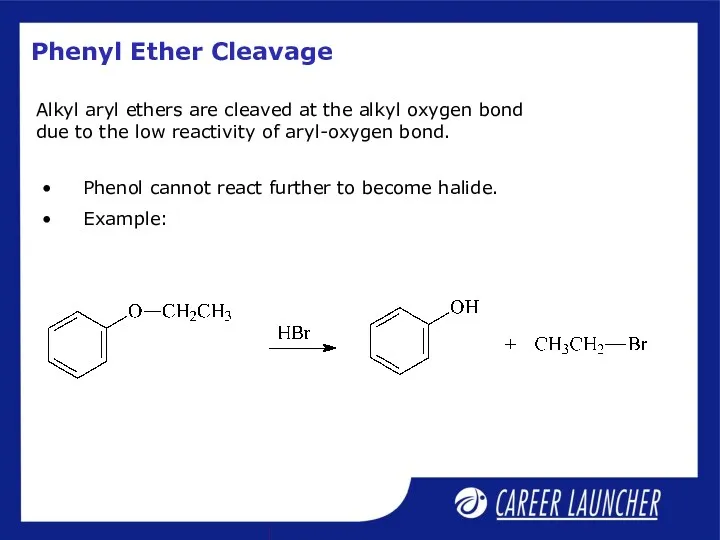
- 26. Phenyl Ether Cleavage Phenol cannot react further to become halide. Example: Alkyl aryl ethers are cleaved
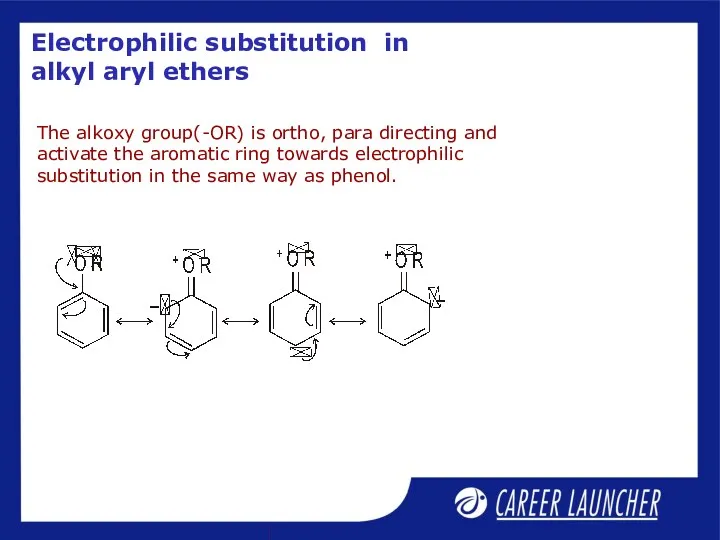
- 27. Electrophilic substitution in alkyl aryl ethers The alkoxy group(-OR) is ortho, para directing and activate the
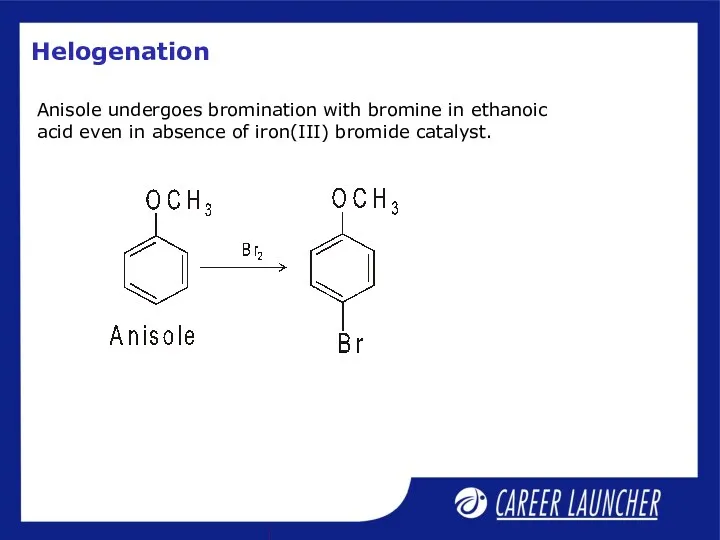
- 28. Helogenation Anisole undergoes bromination with bromine in ethanoic acid even in absence of iron(III) bromide catalyst.
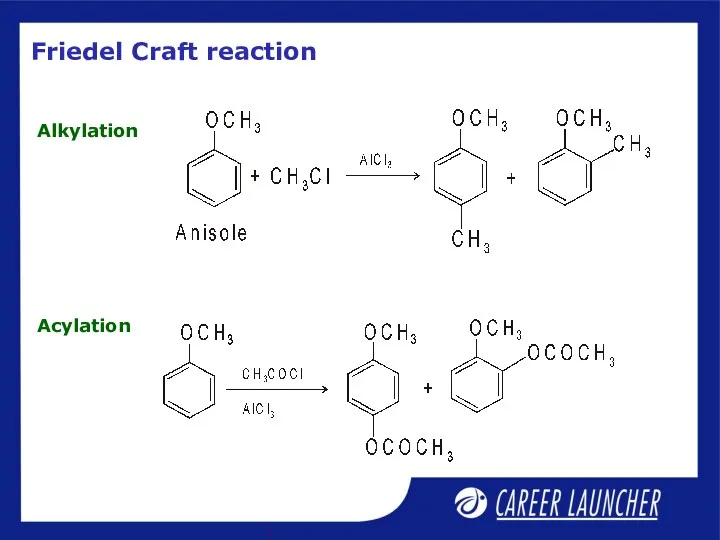
- 29. Friedel Craft reaction Alkylation Acylation
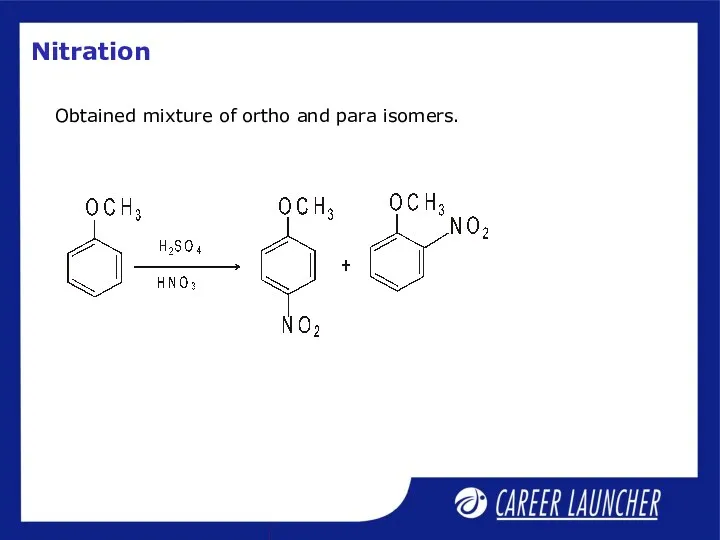
- 30. Nitration Obtained mixture of ortho and para isomers.
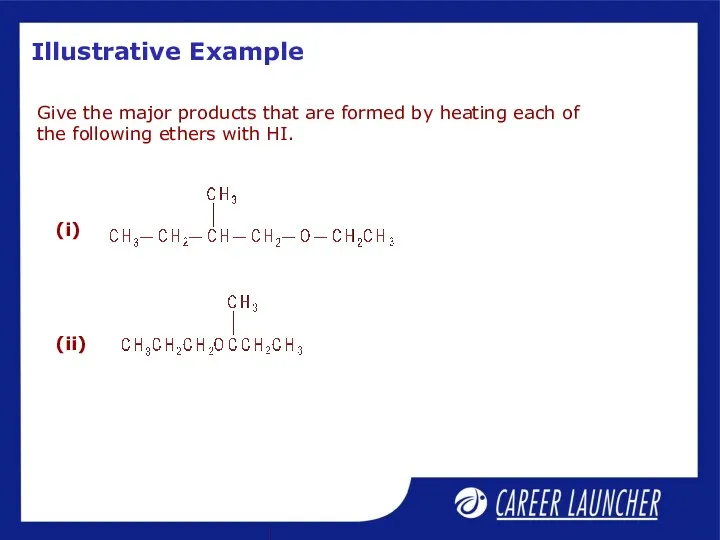
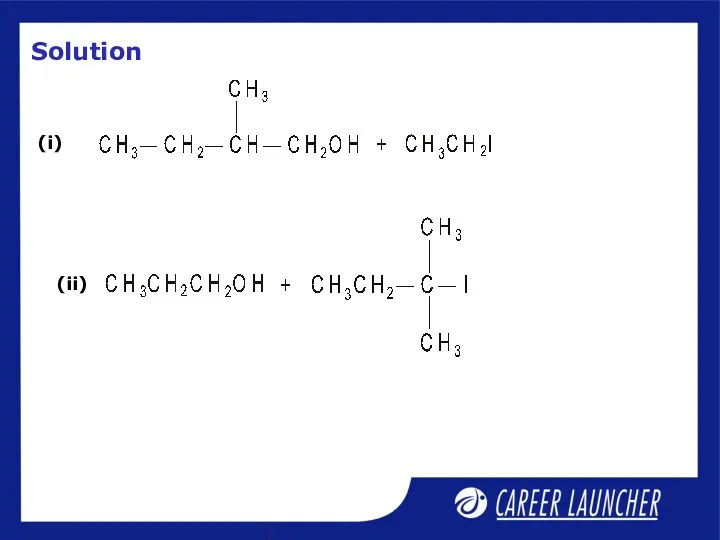
- 31. Illustrative Example
- 32. Solution
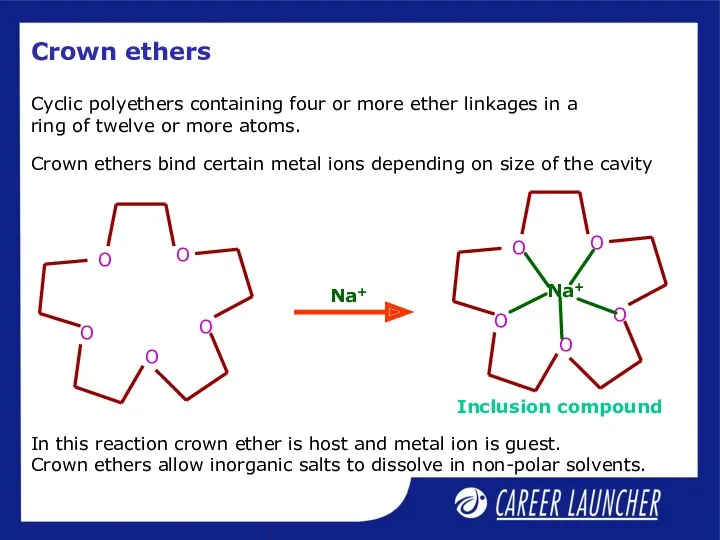
- 33. Crown ethers Cyclic polyethers containing four or more ether linkages in a ring of twelve or
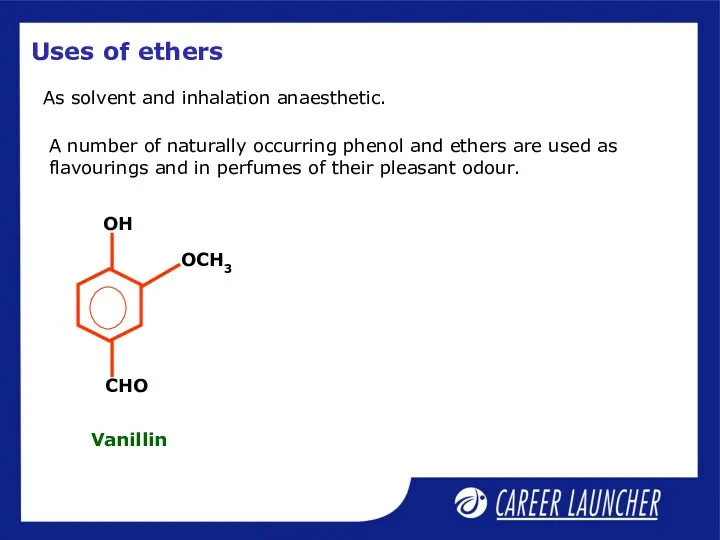
- 34. Uses of ethers As solvent and inhalation anaesthetic. A number of naturally occurring phenol and ethers
- 36. Скачать презентацию


 Липиды, биологическая роль, классификация
Липиды, биологическая роль, классификация Альдегиды и кетоны
Альдегиды и кетоны Кислородсодержащие органические соединения- спирты. 10 класс
Кислородсодержащие органические соединения- спирты. 10 класс Ammonia and amines
Ammonia and amines Химические чистящие средства
Химические чистящие средства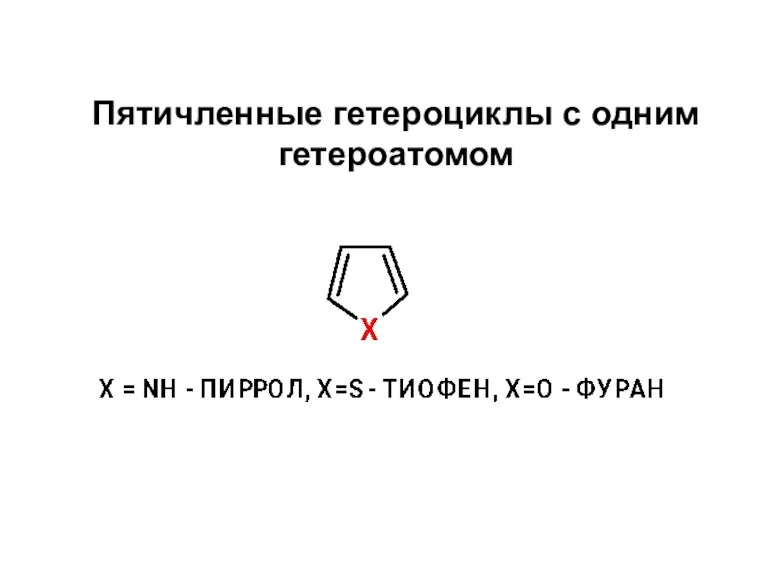 Пятичленные гетероциклы с одним гетероатомом
Пятичленные гетероциклы с одним гетероатомом Обчислення за хімічними рівняннями відносного виходу продукту реакції (11 клас)
Обчислення за хімічними рівняннями відносного виходу продукту реакції (11 клас) Смеси, растворы. Тест
Смеси, растворы. Тест Изотопы, их свойства и применение
Изотопы, их свойства и применение Месторождения нефрита
Месторождения нефрита Чистые вещества и смеси
Чистые вещества и смеси Основания как электролиты, их классификация по различным признакам. Химические свойства оснований
Основания как электролиты, их классификация по различным признакам. Химические свойства оснований 21. Кислоты. Определение кислот
21. Кислоты. Определение кислот Важнейшие оксиды в природе и жизни человека
Важнейшие оксиды в природе и жизни человека Выделение ферментных препаратов методами осаждения и высаливания
Выделение ферментных препаратов методами осаждения и высаливания Волокна. Классификация волокон
Волокна. Классификация волокон Химиялық элементтер
Химиялық элементтер Спирты
Спирты Литий
Литий Стоматологические материалы на основе полимеров
Стоматологические материалы на основе полимеров Основные положения теории растворов электролитов, используемых в аналитической химии
Основные положения теории растворов электролитов, используемых в аналитической химии Розрахункові задачі. Обчислення за хімічними рівняннями. Відносного виходу продукту реакції
Розрахункові задачі. Обчислення за хімічними рівняннями. Відносного виходу продукту реакції Сплавы на основе железа. Диаграмма состояния сплавов системы железо–углерод. Лекция 2. Тема 4
Сплавы на основе железа. Диаграмма состояния сплавов системы железо–углерод. Лекция 2. Тема 4 Строение вещества. Диффузия. Броуновское движение
Строение вещества. Диффузия. Броуновское движение Химиялық тепе-теңдік және оның ығысуына әсер етуші жағдайлар. Ле Шателье-Браун принциптері
Химиялық тепе-теңдік және оның ығысуына әсер етуші жағдайлар. Ле Шателье-Браун принциптері Поверхностные явления на границе раздела фаз. Хроматография, применение в медицинской практике
Поверхностные явления на границе раздела фаз. Хроматография, применение в медицинской практике Учение о растворах
Учение о растворах КЛАССИФИКАЦИЯ ХИМИЧЕСКИХ РЕАКЦИЙ
КЛАССИФИКАЦИЯ ХИМИЧЕСКИХ РЕАКЦИЙ