Содержание
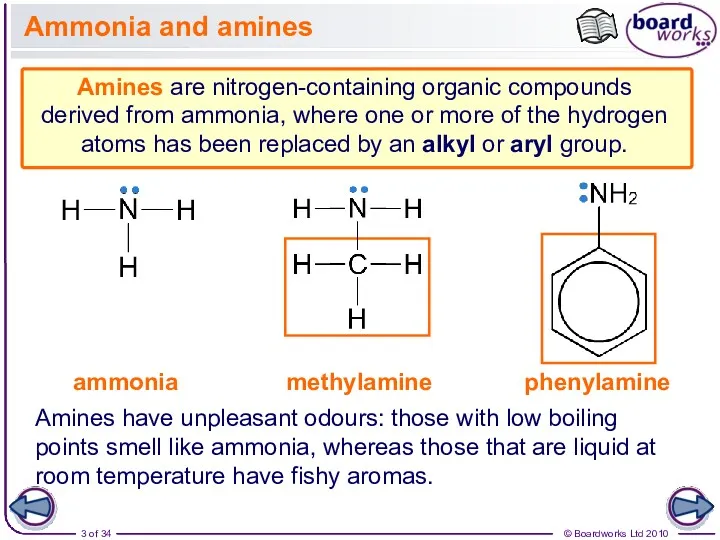
- 3. Ammonia and amines Amines are nitrogen-containing organic compounds derived from ammonia, where one or more of
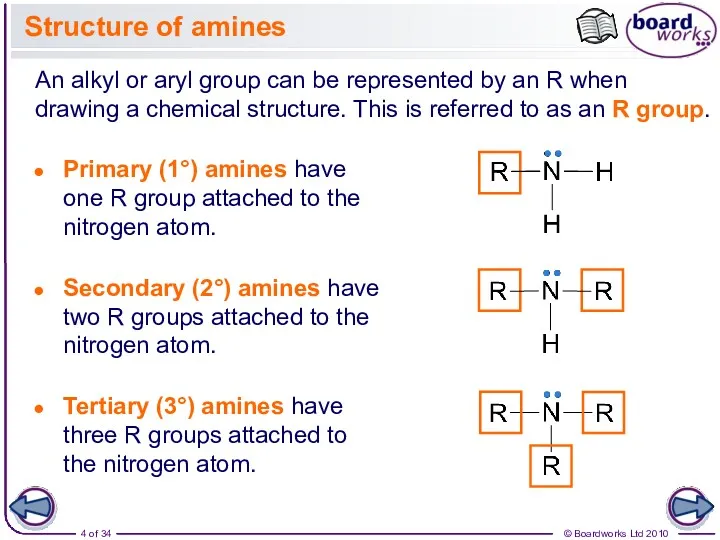
- 4. Structure of amines An alkyl or aryl group can be represented by an R when drawing
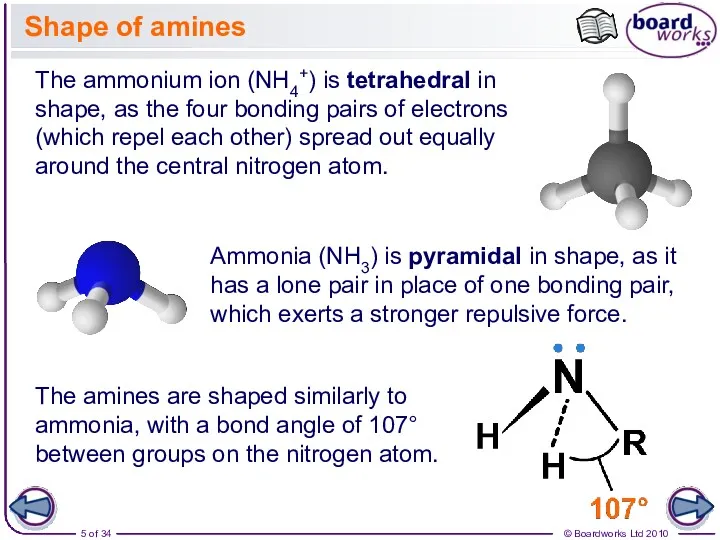
- 5. Shape of amines The ammonium ion (NH4+) is tetrahedral in shape, as the four bonding pairs
- 6. Identifying amines
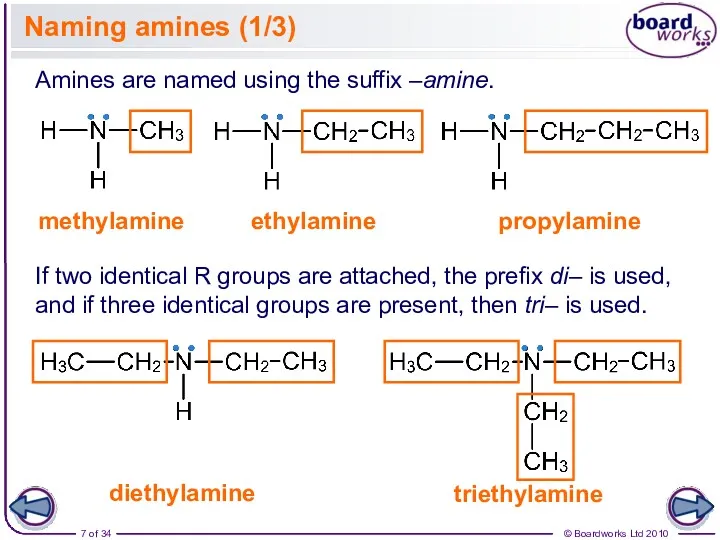
- 7. Naming amines (1/3) Amines are named using the suffix –amine. methylamine ethylamine propylamine diethylamine If two
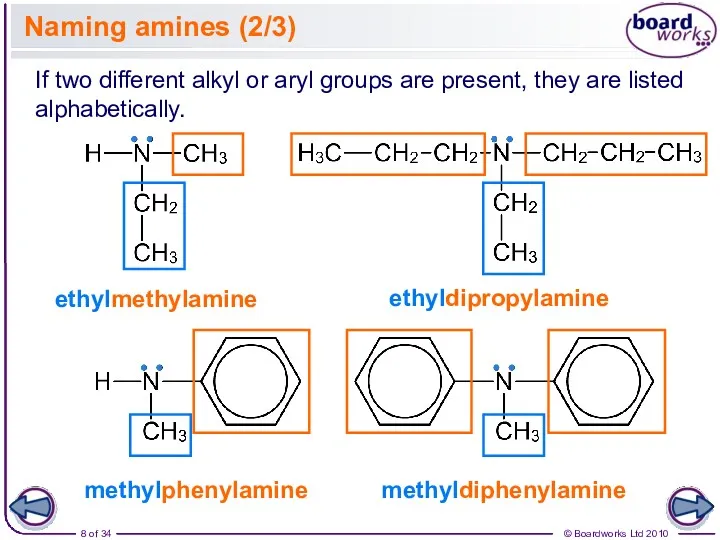
- 8. Naming amines (2/3) ethylmethylamine If two different alkyl or aryl groups are present, they are listed
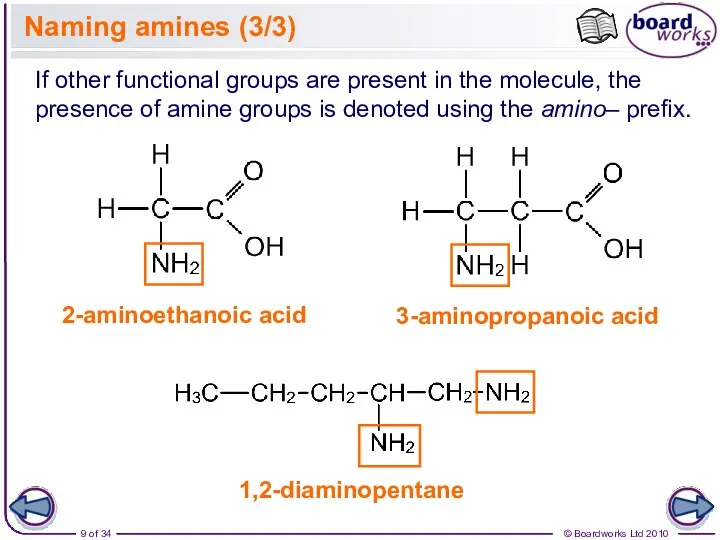
- 9. Naming amines (3/3) 3-aminopropanoic acid If other functional groups are present in the molecule, the presence
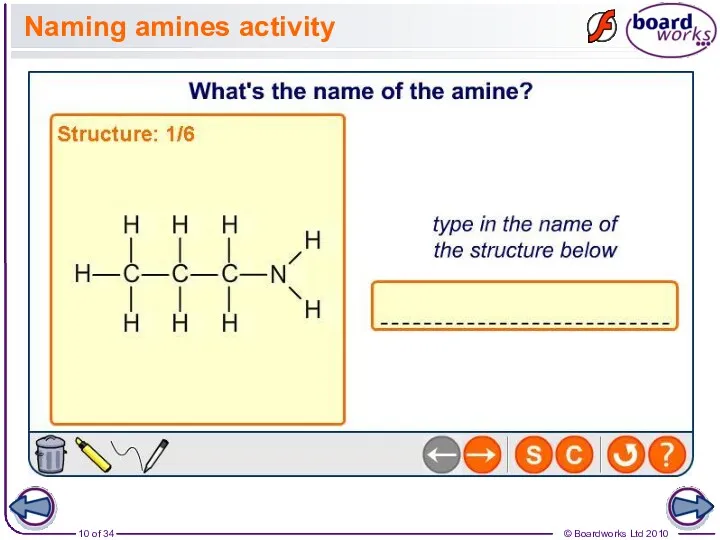
- 10. Naming amines activity
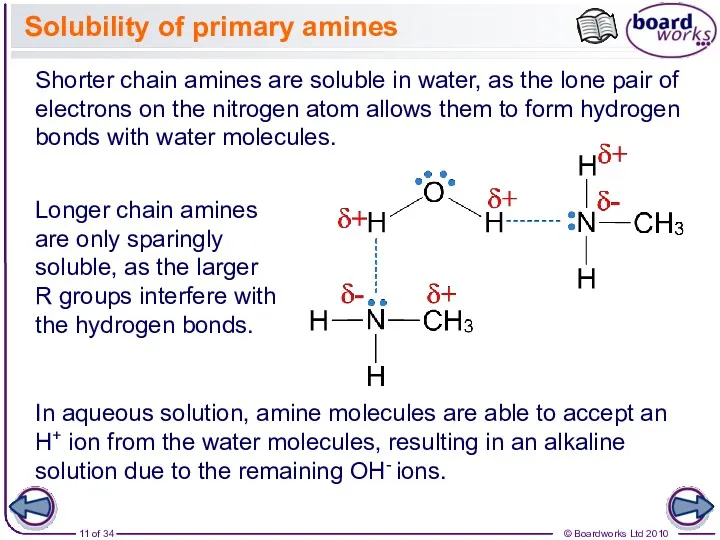
- 11. Solubility of primary amines Longer chain amines are only sparingly soluble, as the larger R groups
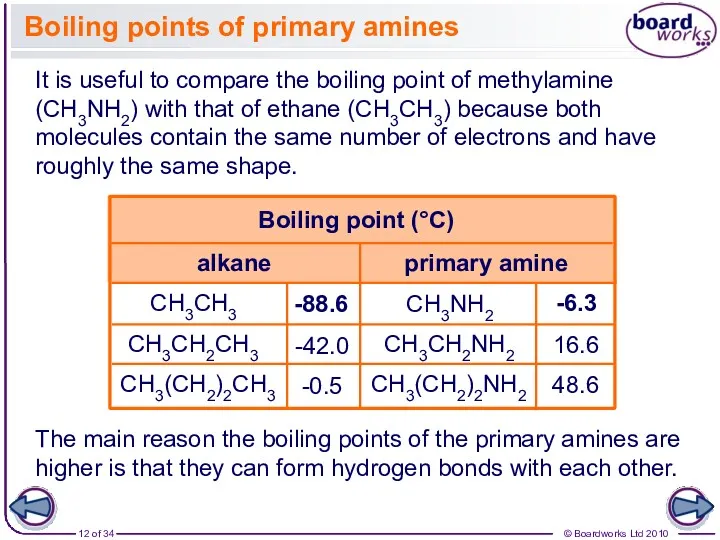
- 12. CH3NH2 CH3CH2NH2 CH3(CH2)2NH2 Boiling points of primary amines It is useful to compare the boiling point
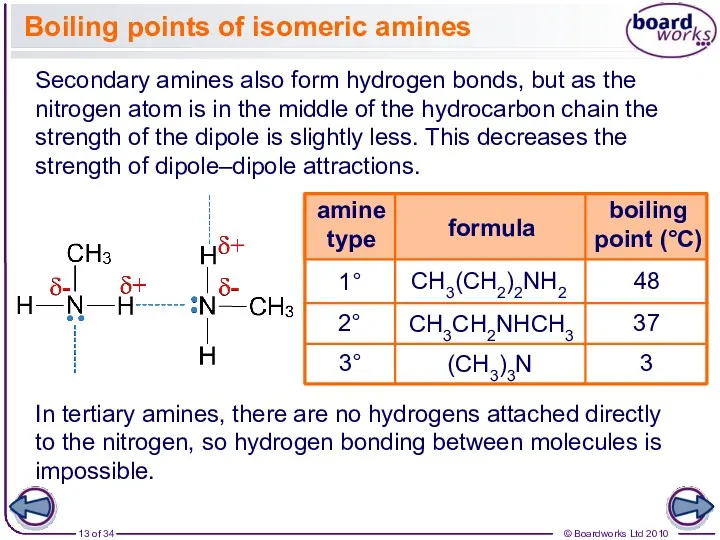
- 13. In tertiary amines, there are no hydrogens attached directly to the nitrogen, so hydrogen bonding between
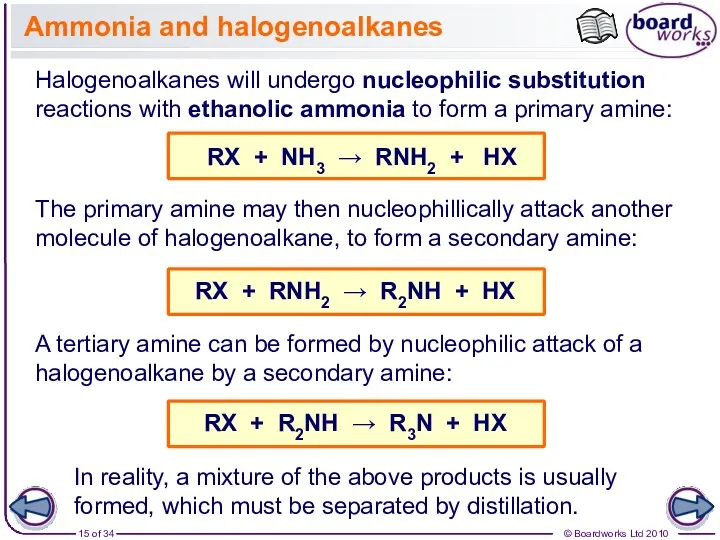
- 15. Ammonia and halogenoalkanes Halogenoalkanes will undergo nucleophilic substitution reactions with ethanolic ammonia to form a primary
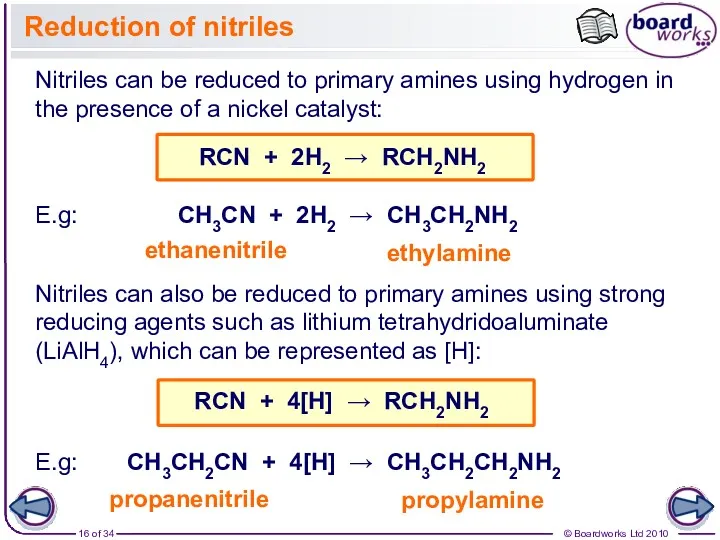
- 16. Reduction of nitriles Nitriles can be reduced to primary amines using hydrogen in the presence of
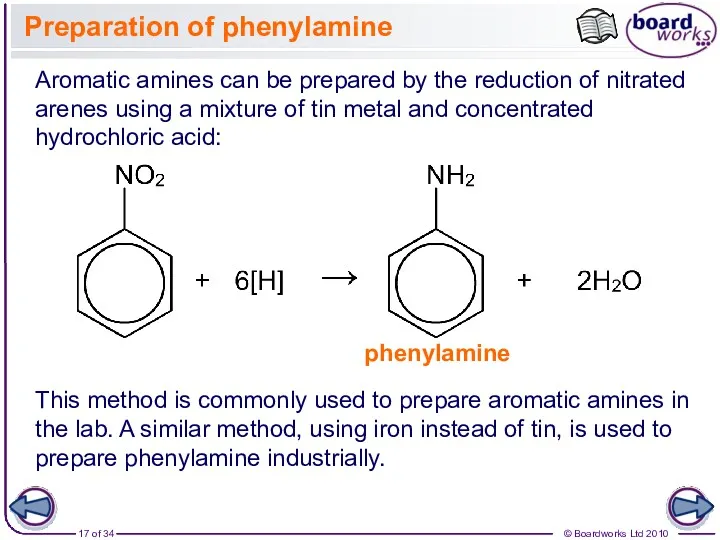
- 17. Preparation of phenylamine Aromatic amines can be prepared by the reduction of nitrated arenes using a
- 18. Which conditions?
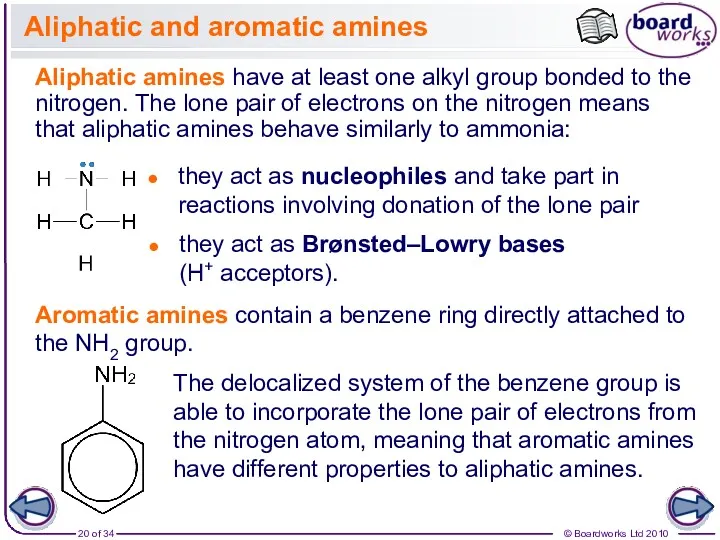
- 20. Aliphatic and aromatic amines Aliphatic amines have at least one alkyl group bonded to the nitrogen.
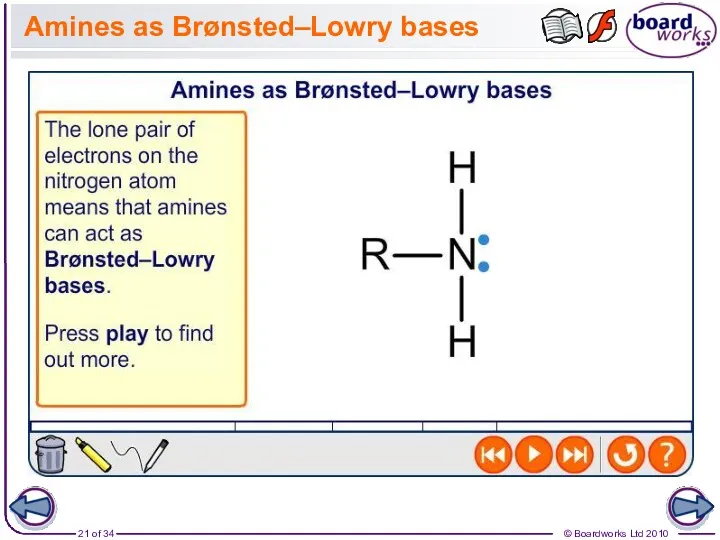
- 21. Amines as Brønsted–Lowry bases
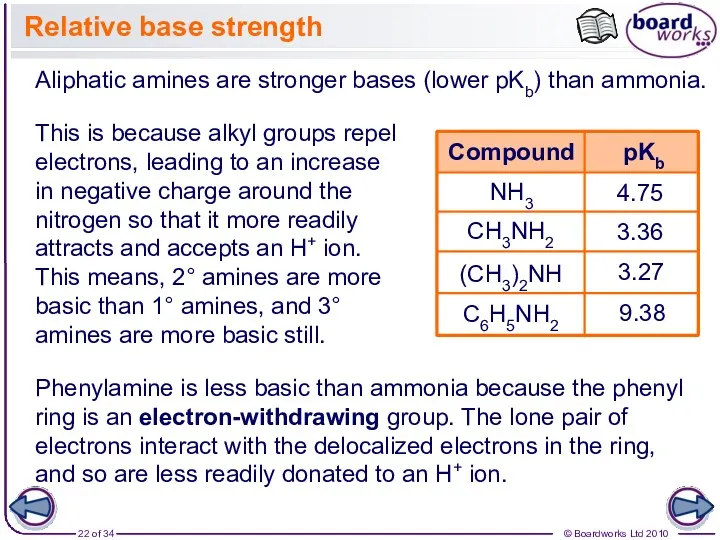
- 22. Relative base strength NH3 4.75 3.36 3.27 Phenylamine is less basic than ammonia because the phenyl
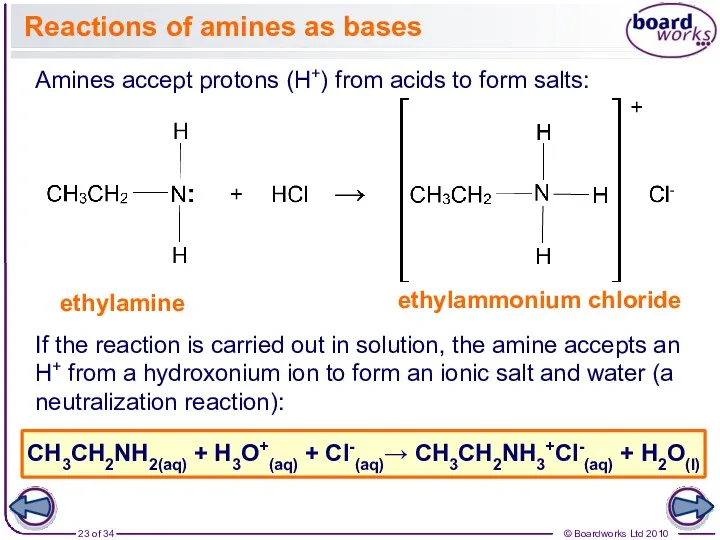
- 23. Reactions of amines as bases Amines accept protons (H+) from acids to form salts: ethylamine ethylammonium
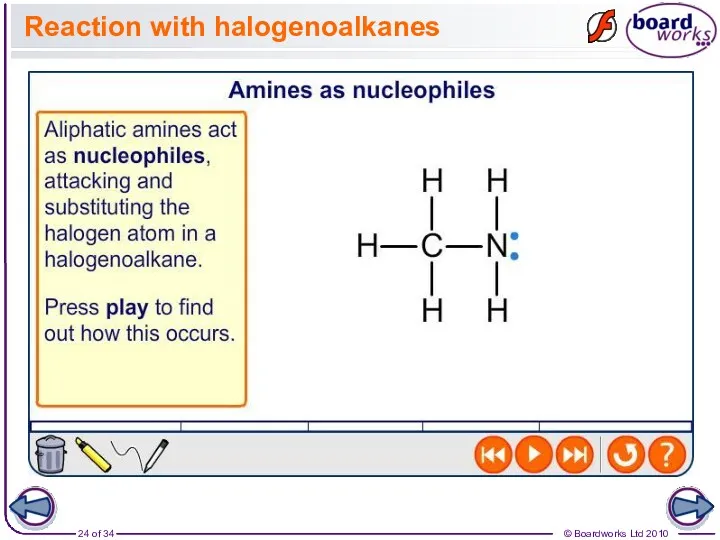
- 24. Reaction with halogenoalkanes
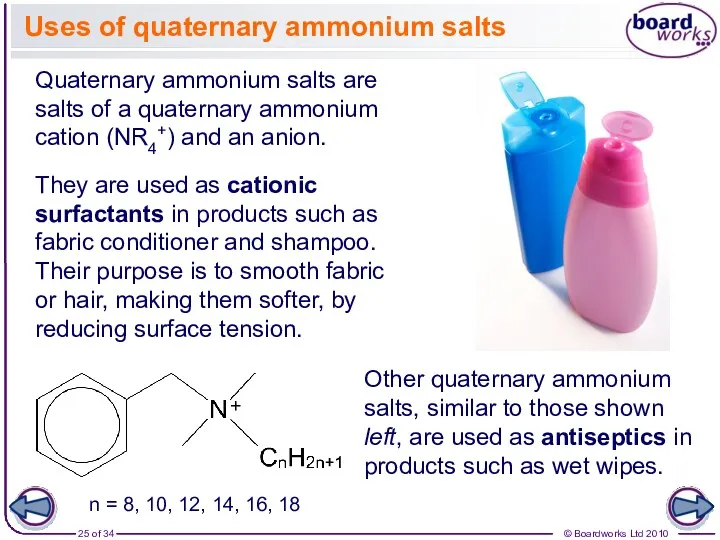
- 25. Uses of quaternary ammonium salts Quaternary ammonium salts are salts of a quaternary ammonium cation (NR4+)
- 26. Reaction with acyl compounds
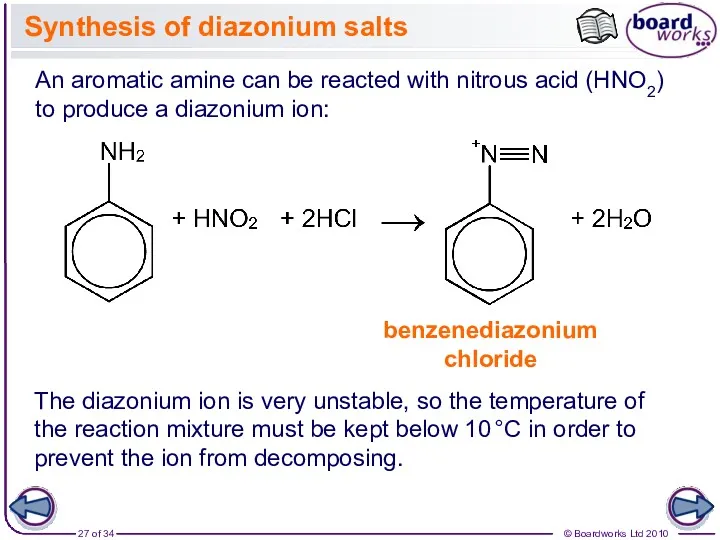
- 27. Synthesis of diazonium salts An aromatic amine can be reacted with nitrous acid (HNO2) to produce
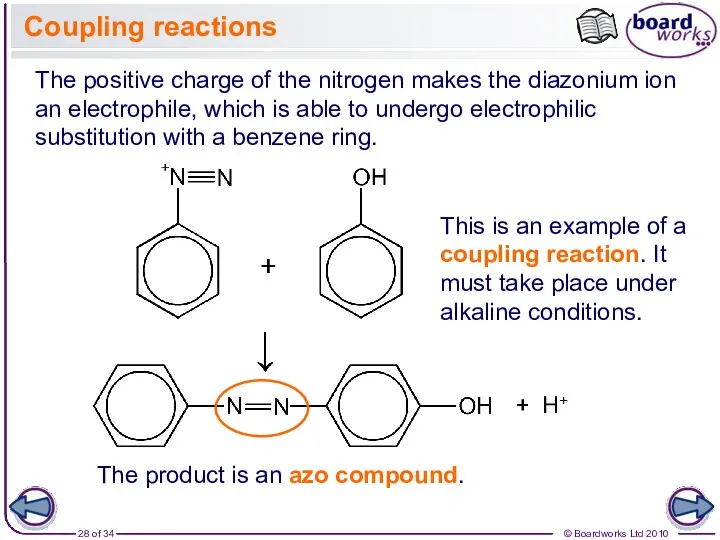
- 28. Coupling reactions The positive charge of the nitrogen makes the diazonium ion an electrophile, which is
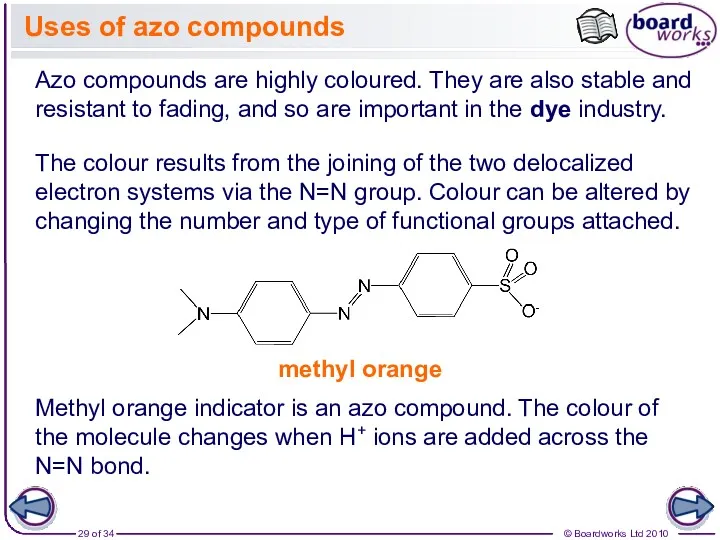
- 29. Uses of azo compounds Azo compounds are highly coloured. They are also stable and resistant to
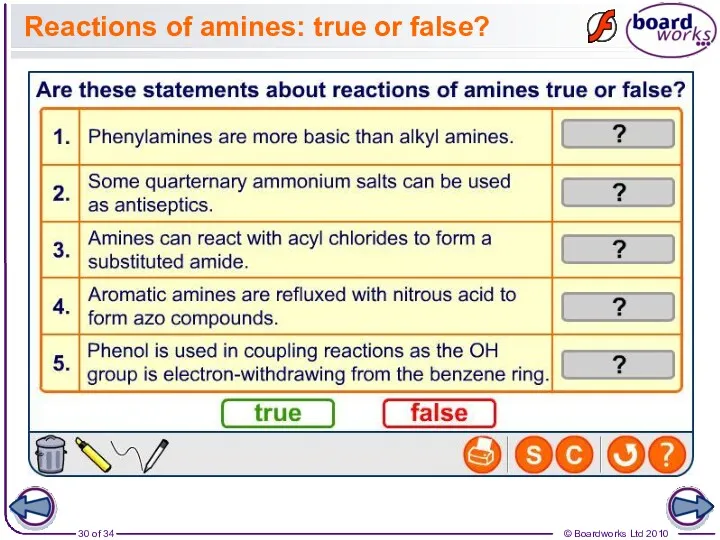
- 30. Reactions of amines: true or false?
- 32. Glossary
- 33. What’s the keyword?
- 35. Скачать презентацию


 Полиметилметакрилат
Полиметилметакрилат Корунд. Разновидности корунда
Корунд. Разновидности корунда Введение в химическую термодинамику
Введение в химическую термодинамику Диссоциация кислот, оснований, солей
Диссоциация кислот, оснований, солей Кристалдардың ішкі құрылымы
Кристалдардың ішкі құрылымы Шкала рН. Лекция 03-1
Шкала рН. Лекция 03-1 Реакции SR в ряду алканов
Реакции SR в ряду алканов Металлы. Общие свойства металлов
Металлы. Общие свойства металлов Беттiк құбылыстар, олардың ағзадағы маңызы. Адсорбция
Беттiк құбылыстар, олардың ағзадағы маңызы. Адсорбция Розчин і його компоненти
Розчин і його компоненти Химические элементы и организм человека
Химические элементы и организм человека Составление формул химических соединений
Составление формул химических соединений Основні класи неорганічних сполук
Основні класи неорганічних сполук Полимеры. Основные понятия
Полимеры. Основные понятия Механизм реакции в органической химии
Механизм реакции в органической химии Водородная связь
Водородная связь Химия нефти и газа. Лекция № 1
Химия нефти и газа. Лекция № 1 Химические реакторы. Гетерогенно-каталитические химические процессы. Лекция №15
Химические реакторы. Гетерогенно-каталитические химические процессы. Лекция №15 Губна помада та ії призначення
Губна помада та ії призначення Теория растворов электролитов и неэлектролитов
Теория растворов электролитов и неэлектролитов Способи очищення води
Способи очищення води Аммиак. Строение молекулы аммиака, его физические и химические свойства
Аммиак. Строение молекулы аммиака, его физические и химические свойства Реакция обменного разложения веществ водой - гидролиз
Реакция обменного разложения веществ водой - гидролиз Метод электронного баланса для уравнивания окислительно-восстановительных реакций
Метод электронного баланса для уравнивания окислительно-восстановительных реакций Моноядерні арени
Моноядерні арени Силіцій
Силіцій Водород
Водород Непредельные углеводороды. Алкены. Номенклатура алкенов
Непредельные углеводороды. Алкены. Номенклатура алкенов