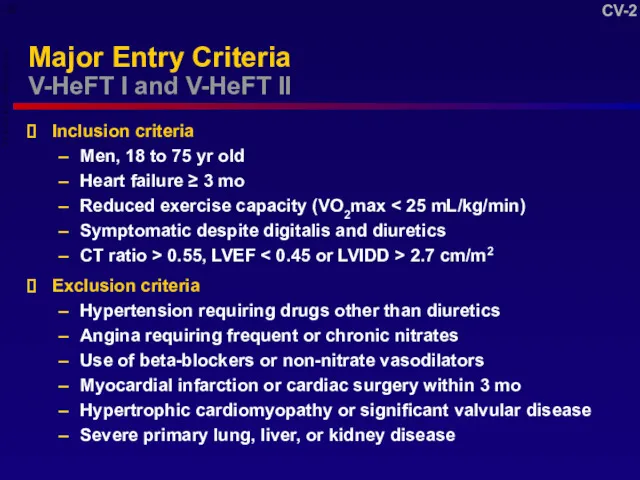
Major Entry Criteria
V-HeFT I and V-HeFT II
Inclusion criteria
Men, 18 to 75
yr old
Heart failure ≥ 3 mo
Reduced exercise capacity (VO2max < 25 mL/kg/min)
Symptomatic despite digitalis and diuretics
CT ratio > 0.55, LVEF < 0.45 or LVIDD > 2.7 cm/m2
Exclusion criteria
Hypertension requiring drugs other than diuretics
Angina requiring frequent or chronic nitrates
Use of beta-blockers or non-nitrate vasodilators
Myocardial infarction or cardiac surgery within 3 mo
Hypertrophic cardiomyopathy or significant valvular disease
Severe primary lung, liver, or kidney disease
26
DV BiDil FDA slides10.ppt

 Желчнокаменная болезнь
Желчнокаменная болезнь Микробтарға қарсы заттар. Антисептикалық және дезинфекциялаушы заттар
Микробтарға қарсы заттар. Антисептикалық және дезинфекциялаушы заттар Диагностика и профилактика стоматологических заболеваний пародонта и роль гигиениста стоматологического
Диагностика и профилактика стоматологических заболеваний пародонта и роль гигиениста стоматологического Болезнь Паркинсона
Болезнь Паркинсона Адренолейкодистрофия
Адренолейкодистрофия Симптоматические гипертонии в амбулаторной практике
Симптоматические гипертонии в амбулаторной практике Алкоголизм, наркомания, токсикомания
Алкоголизм, наркомания, токсикомания Жидкостная цитология в оптимизации цитологической диагностики
Жидкостная цитология в оптимизации цитологической диагностики Скелет конечностей
Скелет конечностей Сестринский уход при различных заболеваниях и состояниях
Сестринский уход при различных заболеваниях и состояниях Анти-акне MESOLAB®
Анти-акне MESOLAB® Амебиаз и его формы
Амебиаз и его формы Ишемический инсульт: расширение показаний для специфической терапии. Тенденции 2016 года
Ишемический инсульт: расширение показаний для специфической терапии. Тенденции 2016 года Соматоформные расстройства. Понятие о соматизации. Этиопатогенез, клиника, терапевтическая тактика
Соматоформные расстройства. Понятие о соматизации. Этиопатогенез, клиника, терапевтическая тактика Патогенные представители семейства энтеробактерий - возбудители шигеллезов, сальмонеллезов. Патогенные кишечные палочки
Патогенные представители семейства энтеробактерий - возбудители шигеллезов, сальмонеллезов. Патогенные кишечные палочки Болезнь Крона
Болезнь Крона Введение в онкологию. Канцерогенез
Введение в онкологию. Канцерогенез Неопухолевые заболевания ободочной кишки. Часть № 2
Неопухолевые заболевания ободочной кишки. Часть № 2 Миоксигенирующий массаж лица
Миоксигенирующий массаж лица Выделительная система. Мочевая система
Выделительная система. Мочевая система Лекция №5. Тема 1.4. Организация и проведение работы в центрах (отделениях) медицинской профилактики, центрах здоровья
Лекция №5. Тема 1.4. Организация и проведение работы в центрах (отделениях) медицинской профилактики, центрах здоровья Субъективные, объективные, инструментальные и лабораторные методы исследования пациентов
Субъективные, объективные, инструментальные и лабораторные методы исследования пациентов Ампулаларды этикеттеу. Ампулалар өндірісінде кешенді механизациялау және автоматтандыру проблемалары
Ампулаларды этикеттеу. Ампулалар өндірісінде кешенді механизациялау және автоматтандыру проблемалары Аномалии родовой деятельности
Аномалии родовой деятельности Мутационная теория онкогенеза
Мутационная теория онкогенеза Клиническая фармакология наркотических анальгетиков
Клиническая фармакология наркотических анальгетиков Клеточный, гуморальный иммунитет и их роль в защите от инфекций
Клеточный, гуморальный иммунитет и их роль в защите от инфекций Виразкова хвороба у дітей
Виразкова хвороба у дітей