Содержание
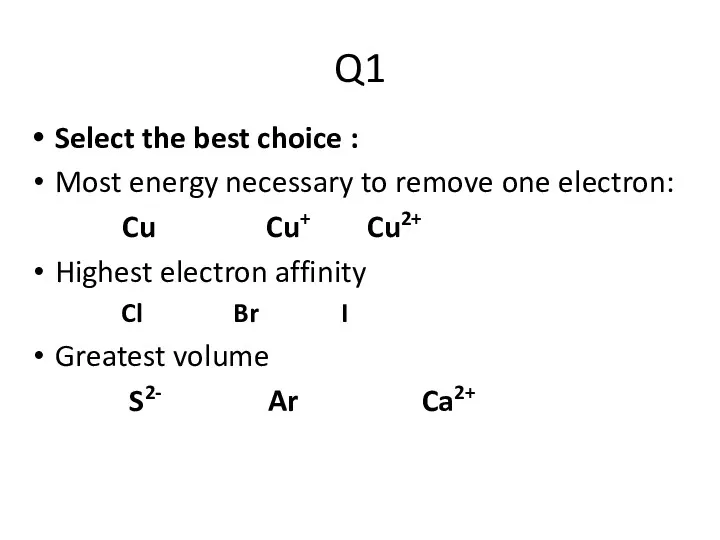
- 2. Q1 Select the best choice : Most energy necessary to remove one electron: Cu Cu+ Cu2+
- 3. R1 Select the best choice : Most energy necessary to remove one electron: Cu Cu+ Cu2+
- 4. Q2 Explain why Ag+ is the most common ion for silver. Which is the more likely
- 5. R2 Ag+ is the most common ion for silver because it has [Kr]4d10 . With filled
- 6. R1C The preferred configuration of Mn2+ is [Ar]3d5 The 3d orbital are lower in energy than
- 7. 3.2 Units A) Electromagnetic Radiation 3.2.1: Electromagnetic Radiation 3.2.2: Quantization 3.2.3: The Atomic Spectrum of Hydrogen
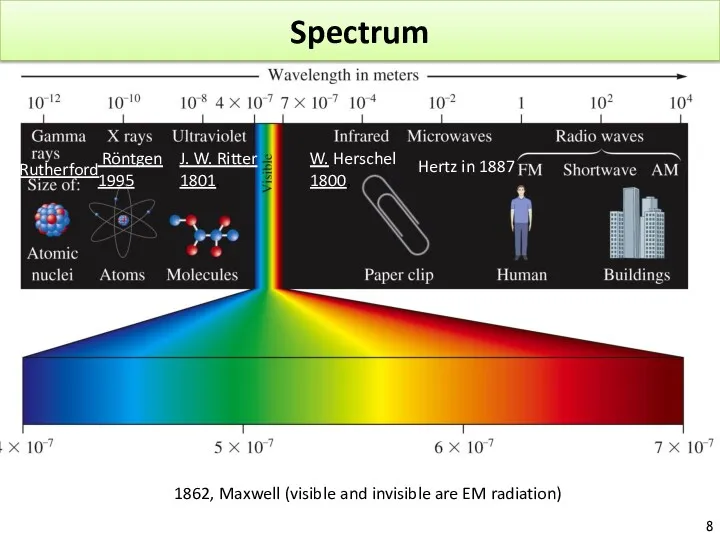
- 8. Spectrum 1862, Maxwell (visible and invisible are EM radiation) J. W. Ritter 1801, W. Herschel 1800,
- 9. ELECTROMAGNETIC RADIATION
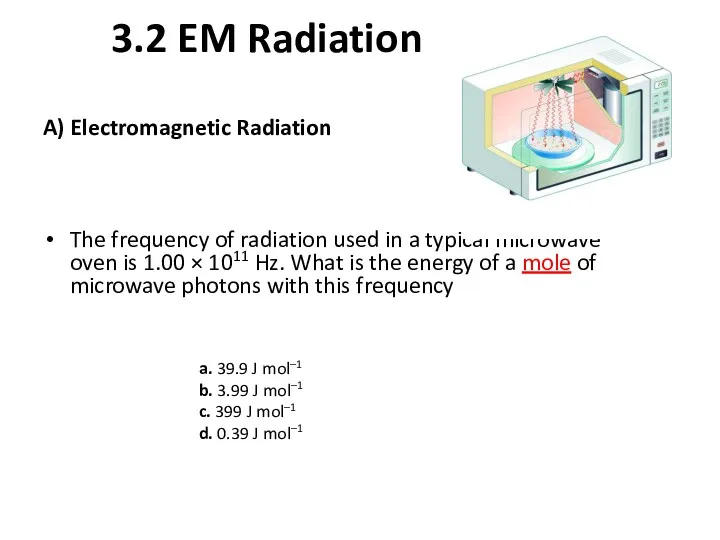
- 10. 3.2 EM Radiation A) Electromagnetic Radiation The frequency of radiation used in a typical microwave oven
- 11. 3.2 Units A) Electromagnetic Radiation The frequency of radiation used in a typical microwave oven is
- 12. Solution 1 E = hν, Multiply this value by the Avogadro constant to find the energy
- 13. Solution a. 39.9 J mol–1 b. 3.99 J mol–1 c. 399 J mol–1 d. 0.39 J
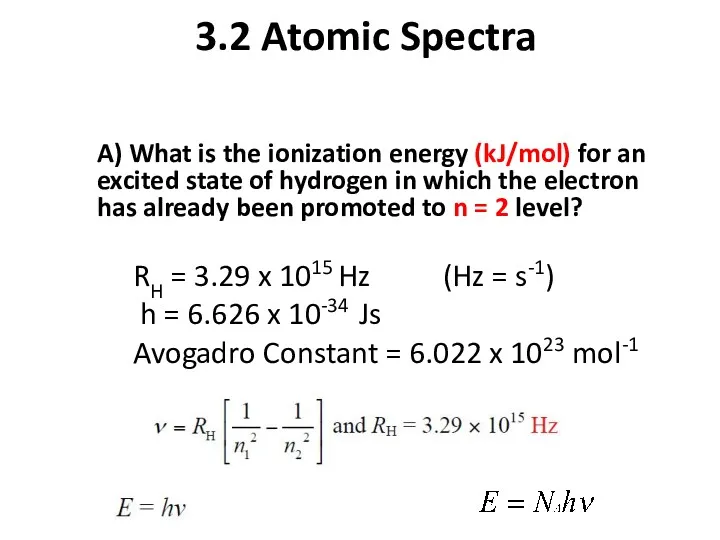
- 14. 3.2 Atomic Spectra A) What is the ionization energy (kJ/mol) for an excited state of hydrogen
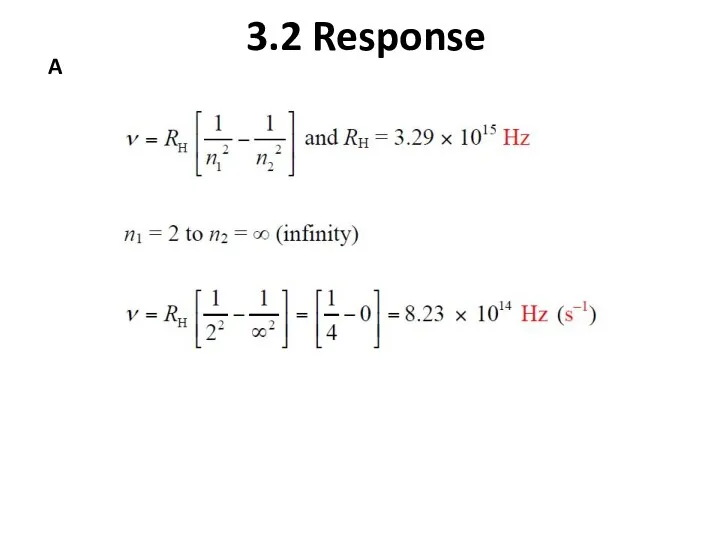
- 15. 3.2 Response A
- 16. 3.2 Response A
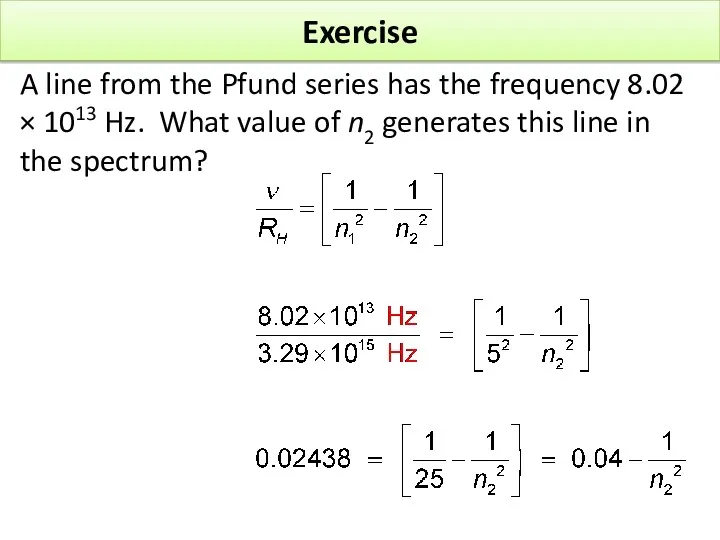
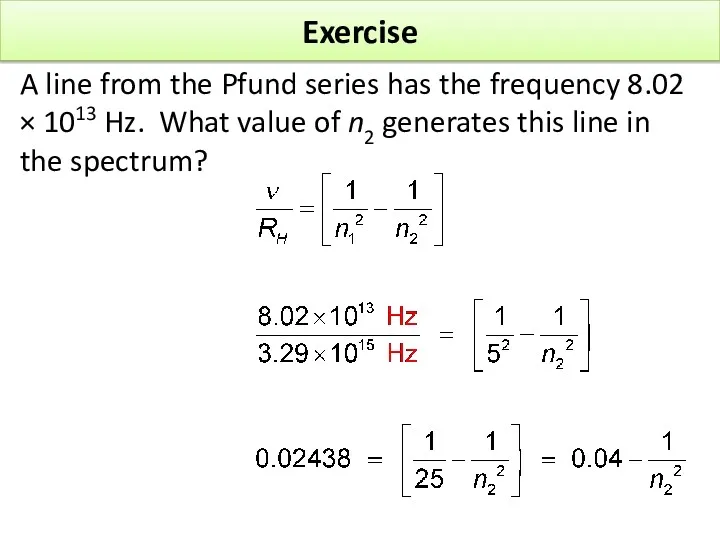
- 17. Exercise A line from the Pfund series has the frequency 8.02 × 1013 Hz. What value
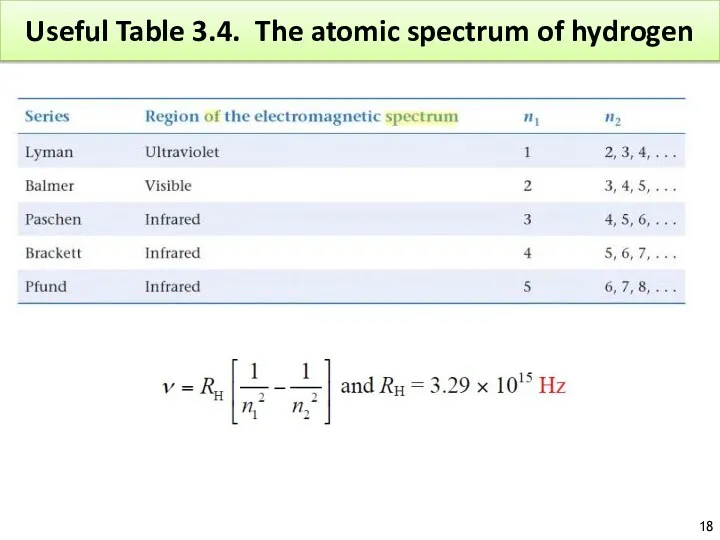
- 18. Useful Table 3.4. The atomic spectrum of hydrogen
- 19. Exercise A line from the Pfund series has the frequency 8.02 × 1013 Hz. What value
- 20. Exercise A line from the Pfund series has the frequency 8.02 × 1013 Hz. What value
- 21. Exercise Rearranging, by taking 0.04 from each side, gives Dividing both sides by –0.01563 and multiplying
- 22. 3.2 Light Interference In Thomas Young’s experiment when he passed light through two closely placed slits,
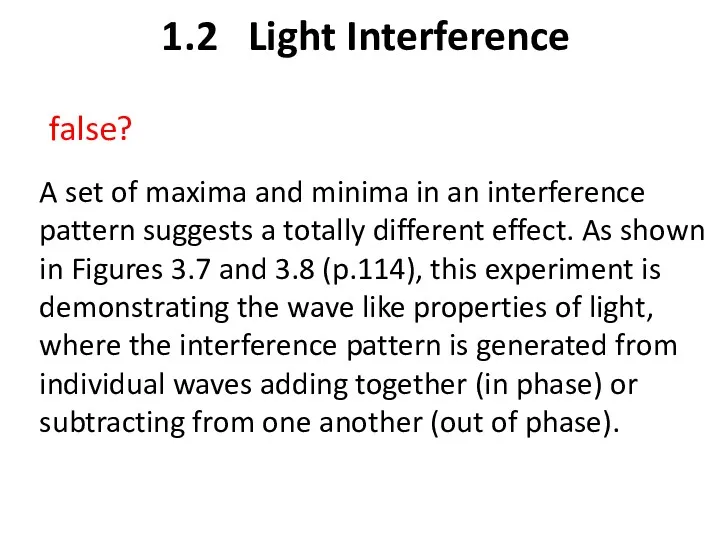
- 23. 1.2 Light Interference A set of maxima and minima in an interference pattern suggests a totally
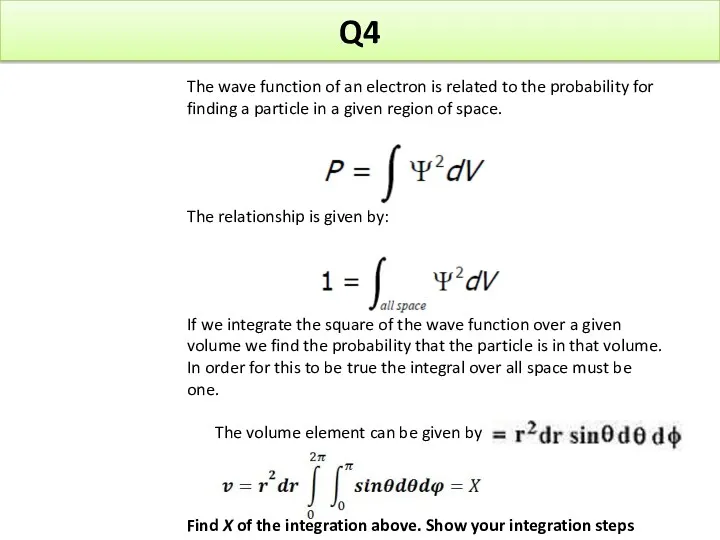
- 24. Q4 The wave function of an electron is related to the probability for finding a particle
- 25. Radial Probability Distribution https://www.youtube.com/watch?v=Prf_jzbD_bM Ψ(r, θ, φ)=R(r) Y(θ, φ)
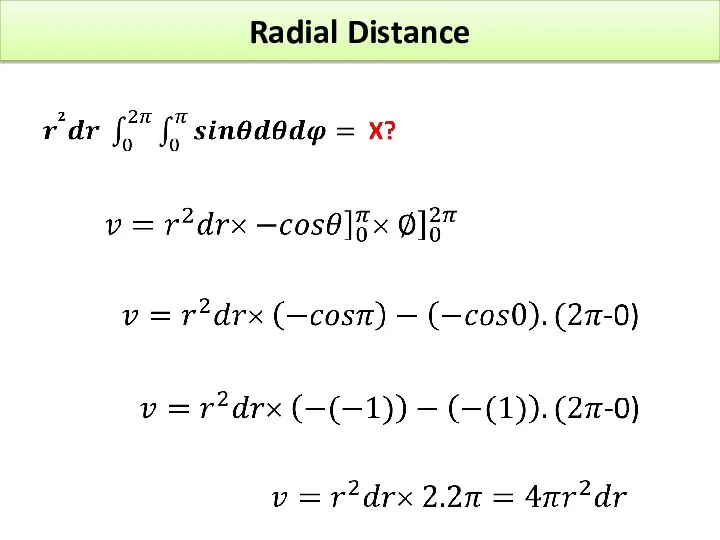
- 26. Radial Distance
- 27. Exercise Sketch radial wavefunctions, radial distribution functions and boundary diagrams for 6s and 5p electrons
- 28. Radial nodes for S = n-1 Radial nodes for p = n-2
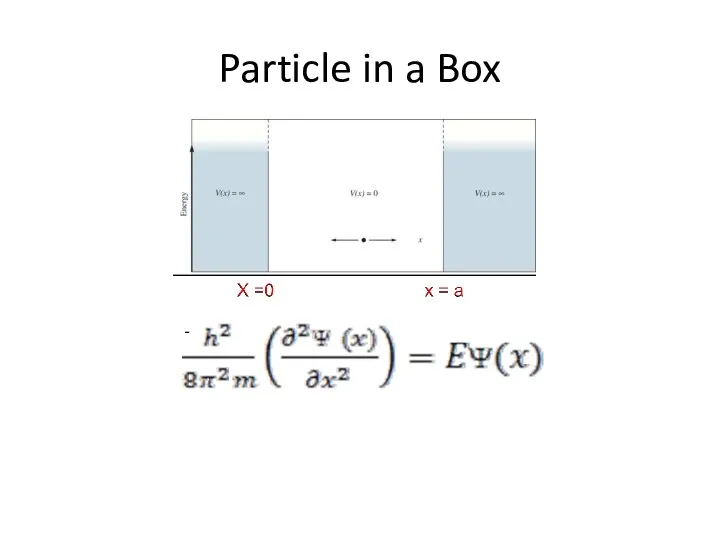
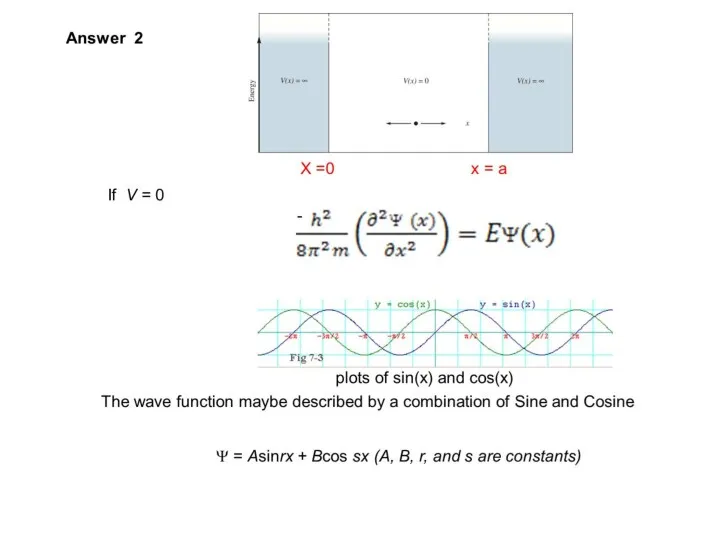
- 29. Particle in a Box
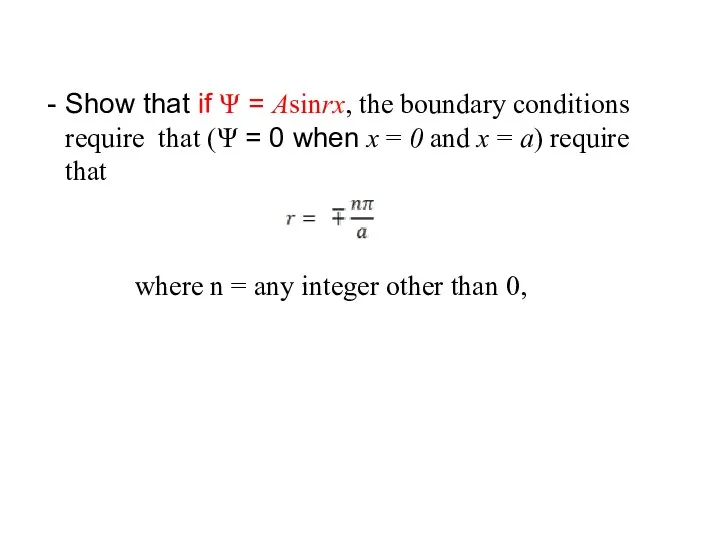
- 33. Show that if Ψ = Asinrx, the boundary conditions require that (Ψ = 0 when x
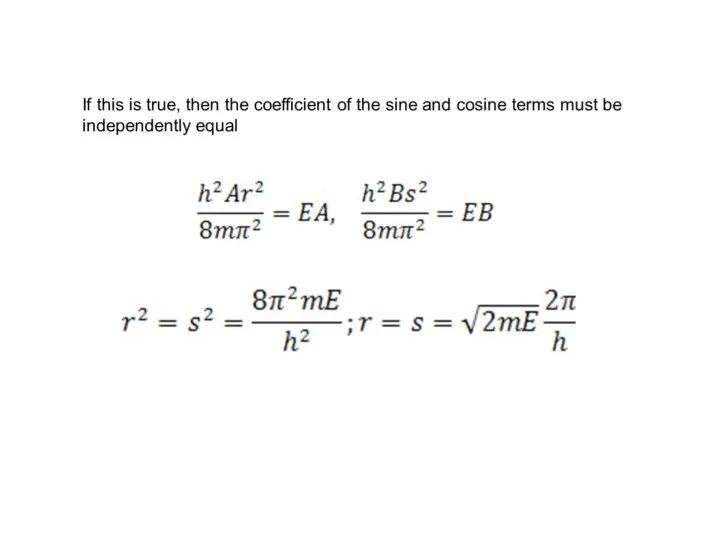
- 35. Show that if , the energy levels of the particle are given by
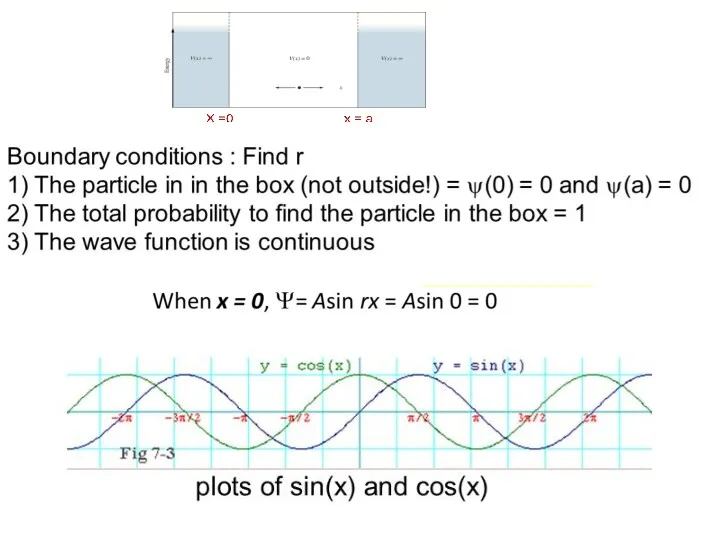
- 37. Show that substituting the value of r given in question C into ψ=Asinrx and applying the
- 39. Integration
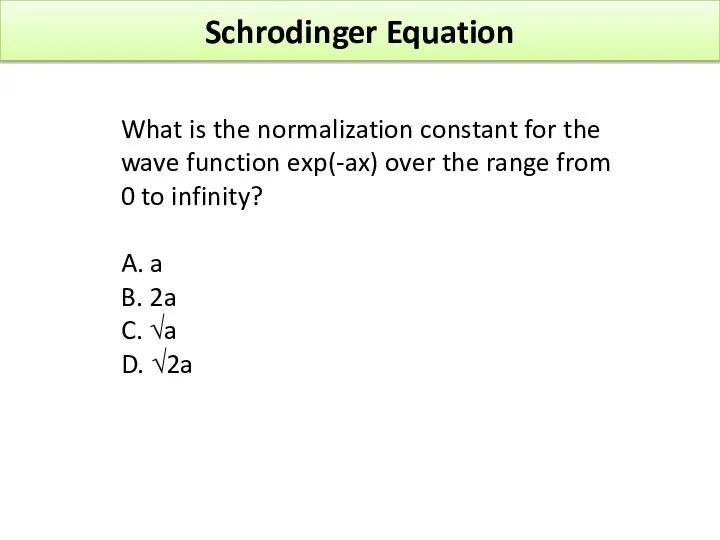
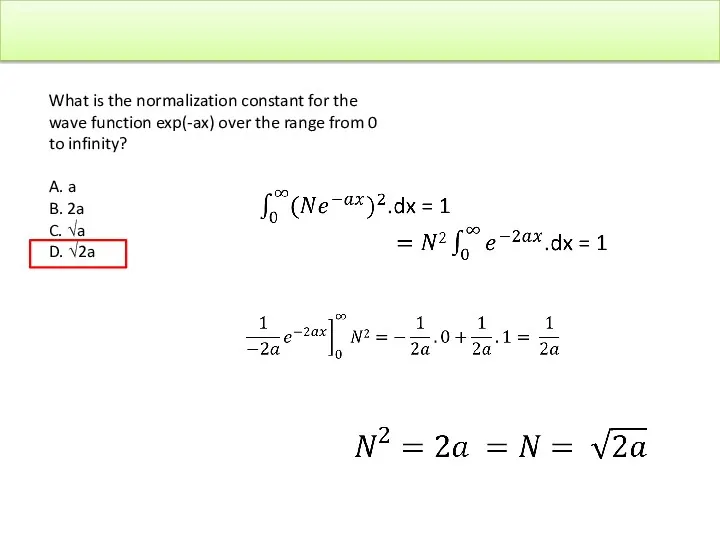
- 40. Schrodinger Equation What is the normalization constant for the wave function exp(-ax) over the range from
- 41. What is the normalization constant for the wave function exp(-ax) over the range from 0 to
- 42. Match the type of orbital defined by the quantum numbers given in questions (a) to (d)
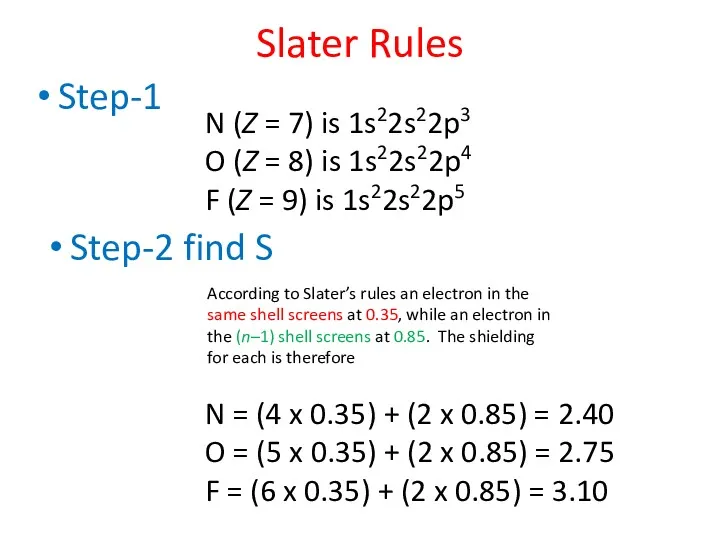
- 43. Use Slater’s rules to determine the relative sizes of N, O and F atoms. Slater Rules
- 44. Step-1 Slater Rules N (Z = 7) is 1s22s22p3 O (Z = 8) is 1s22s22p4 F
- 45. Use Slater’s rules to determine the relative sizes of N, O and F atoms. Slater Rules
- 46. Electronic Configuration
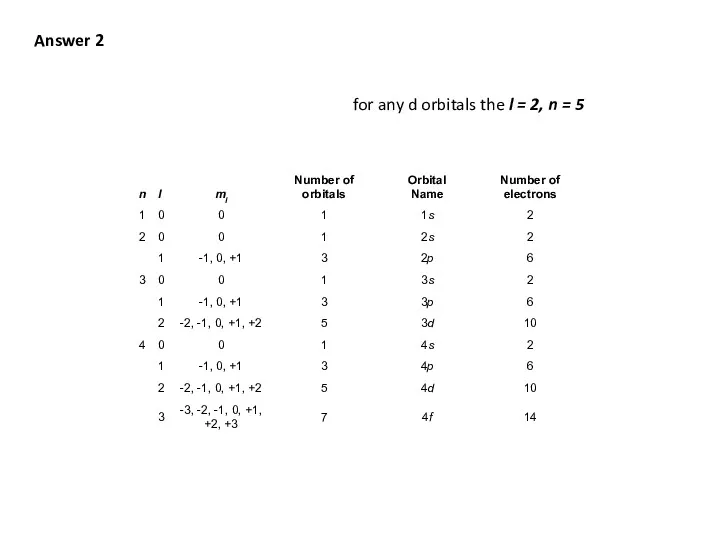
- 47. Q3 What are the values and quantum numbers l and n of a 5d electron. n
- 48. Answer 2 for any d orbitals the l = 2, n = 5
- 49. R1c Explain factors that cause lanthanide contraction. (0.25pts) Explain why Ag+ is the most common ion
- 50. ρsinφ Δρ Angle = Arc Length radius of the circle Arc Length × Arc Length ×
- 52. Скачать презентацию



![R1C The preferred configuration of Mn2+ is [Ar]3d5 The 3d](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/262008/slide-5.jpg)
























 Алкадиены, нафтены
Алкадиены, нафтены Аминокислоты
Аминокислоты Биологически важные пяти- и шестичленные гетероциклы с одним и двумя гетероатомами
Биологически важные пяти- и шестичленные гетероциклы с одним и двумя гетероатомами Серная кислота
Серная кислота Фізичні та хімічні властивості кислот (урок хімії у 8 класі)
Фізичні та хімічні властивості кислот (урок хімії у 8 класі) Типы химических реакций , признаки и условия их протекания
Типы химических реакций , признаки и условия их протекания Физико-химия полимеров и их растворов
Физико-химия полимеров и их растворов Азот
Азот Хімічні властивості кислот
Хімічні властивості кислот Поширення солей у природі
Поширення солей у природі Химический потенциал. Фазовые равновесия
Химический потенциал. Фазовые равновесия ПЛАСТИК НОВЫЙ
ПЛАСТИК НОВЫЙ Stirring in liquid media
Stirring in liquid media Литий
Литий Липиды (Жиры)
Липиды (Жиры) В чём соль соли
В чём соль соли Классификация химических реакций по различным основаниям. 9 класс
Классификация химических реакций по различным основаниям. 9 класс Технологии получения полимерных нанокомпозитов
Технологии получения полимерных нанокомпозитов Гетерофазный катализ. (Лекция 20)
Гетерофазный катализ. (Лекция 20) Правила роботи на уроці
Правила роботи на уроці Задачи на процентную концентрацию
Задачи на процентную концентрацию Углероды. Строение и свойства атомов
Углероды. Строение и свойства атомов Спирт µндіру технологиясы
Спирт µндіру технологиясы Решение заданий по теме: Оксиды
Решение заданий по теме: Оксиды Основная. Первоначальные представления об органических веществах
Основная. Первоначальные представления об органических веществах Химические свойства серной кислоты
Химические свойства серной кислоты Никель қаптамаларын алу жолдары
Никель қаптамаларын алу жолдары Полистирол өндірісі
Полистирол өндірісі