Содержание
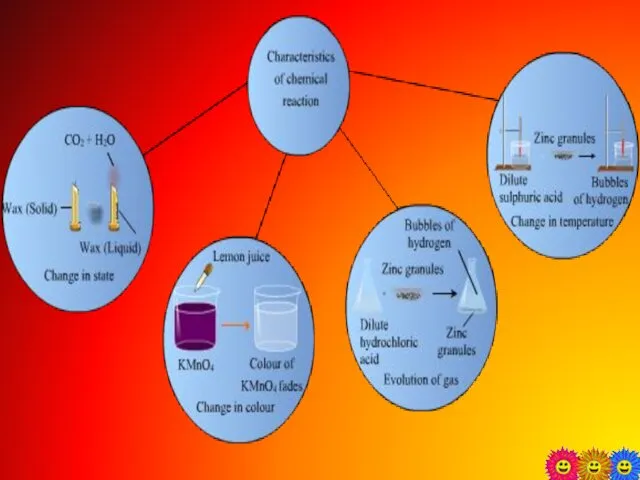
- 2. Chemical changes The formation of new substances takes place with different chemical properties is called chemical
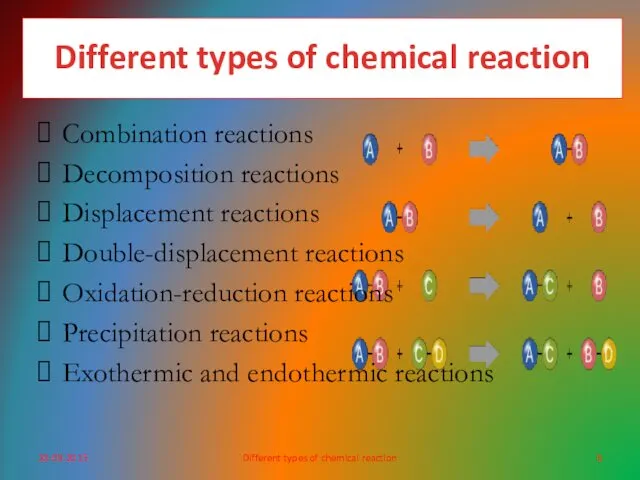
- 4. Different types of chemical reaction 30-09-2015
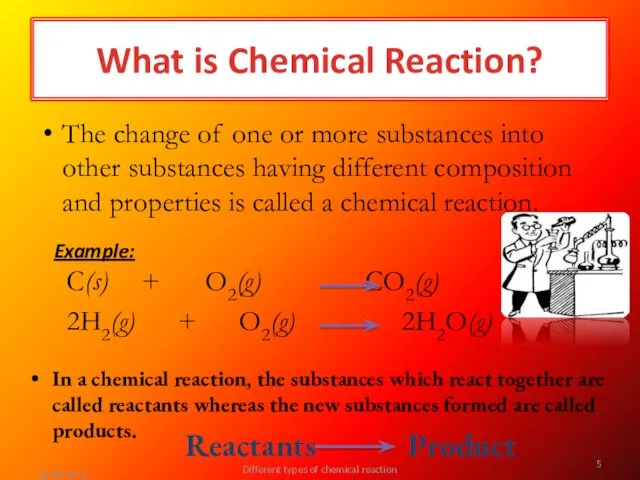
- 5. In a chemical reaction, the substances which react together are called reactants whereas the new substances
- 6. Different types of chemical reaction Combination reactions Decomposition reactions Displacement reactions Double-displacement reactions Oxidation-reduction reactions Precipitation
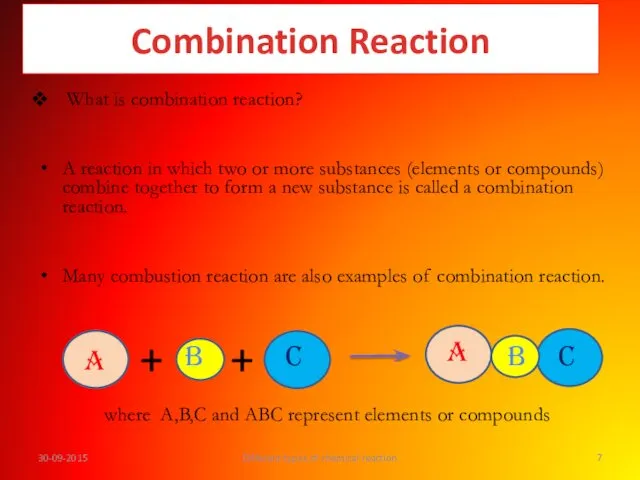
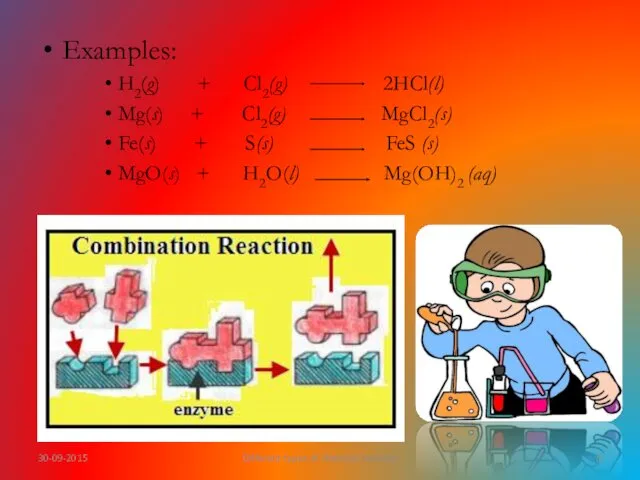
- 7. What is combination reaction? A reaction in which two or more substances (elements or compounds) combine
- 8. Examples: H2(g) + Cl2(g) 2HCl(l) Mg(s) + Cl2(g) MgCl2(s) Fe(s) + S(s) FeS (s) MgO(s) +
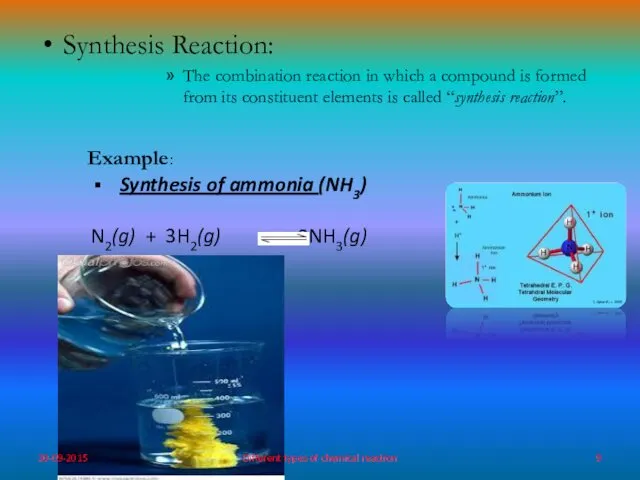
- 9. Synthesis Reaction: The combination reaction in which a compound is formed from its constituent elements is
- 10. What is decomposition reaction? A reaction in which a substance is broken down into two or
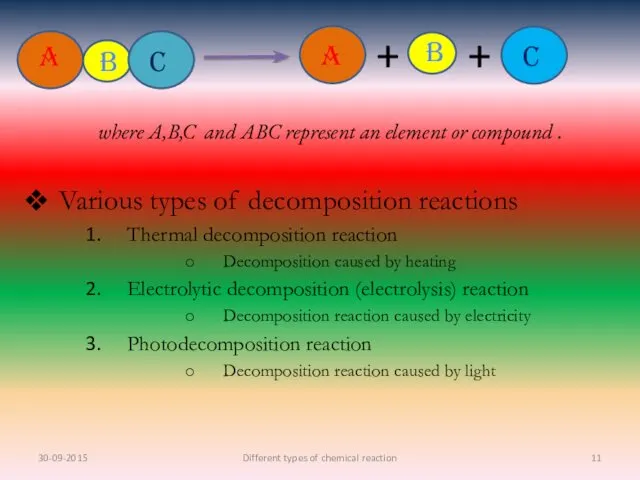
- 11. Various types of decomposition reactions Thermal decomposition reaction Decomposition caused by heating Electrolytic decomposition (electrolysis) reaction
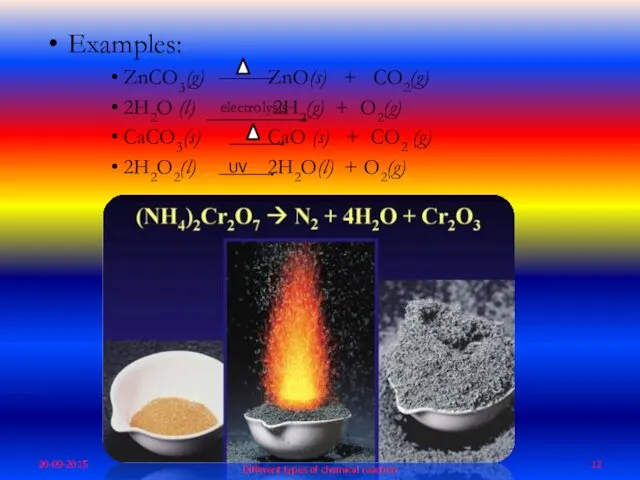
- 12. Examples: ZnCO3(g) ZnO(s) + CO2(g) 2H2O (l) 2H2(g) + O2(g) CaCO3(s) CaO (s) + CO2 (g)
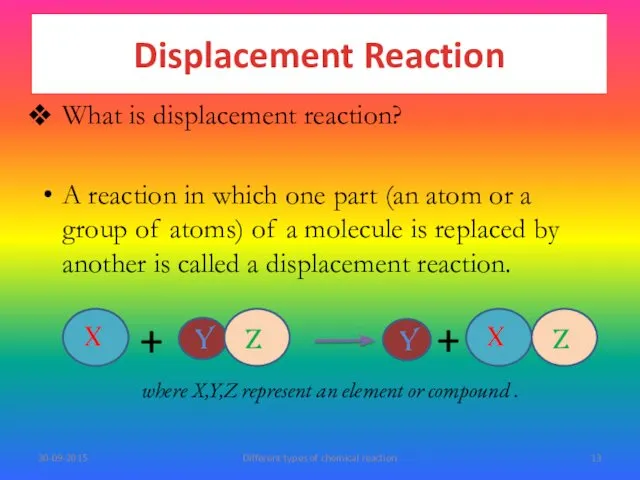
- 13. What is displacement reaction? A reaction in which one part (an atom or a group of
- 14. Examples: Zn(s) + 2HCl(dil) ZnCl2(aq) + H2(g) 2KBr(aq) + Cl2(aq) 2KCl(aq) + Br2(aq) CuSO4(aq) + Zn(s)
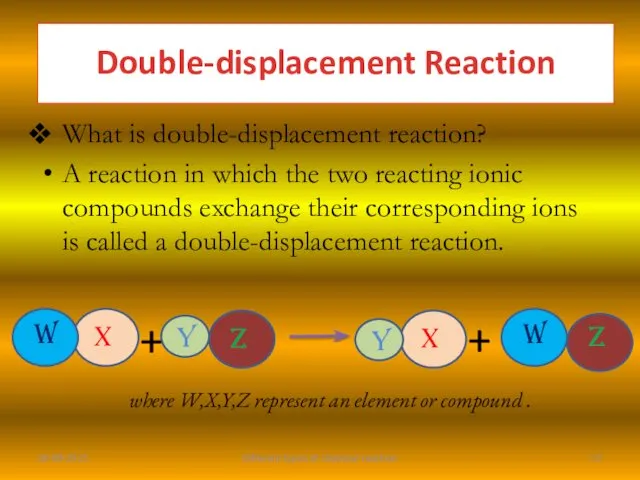
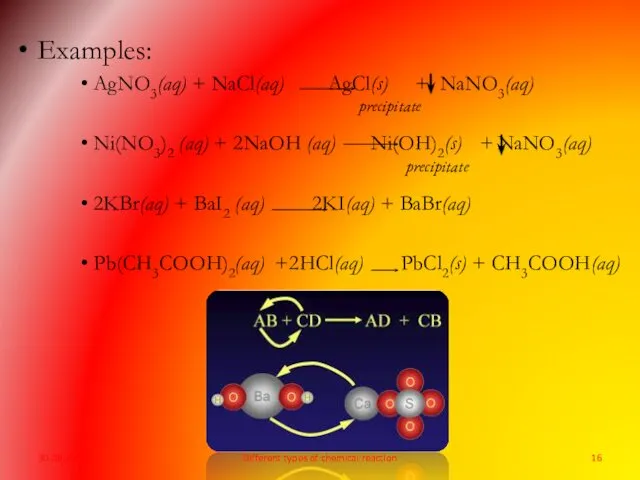
- 15. What is double-displacement reaction? A reaction in which the two reacting ionic compounds exchange their corresponding
- 16. Examples: AgNO3(aq) + NaCl(aq) AgCl(s) + NaNO3(aq) Ni(NO3)2 (aq) + 2NaOH (aq) Ni(OH)2(s) + NaNO3(aq) 2KBr(aq)
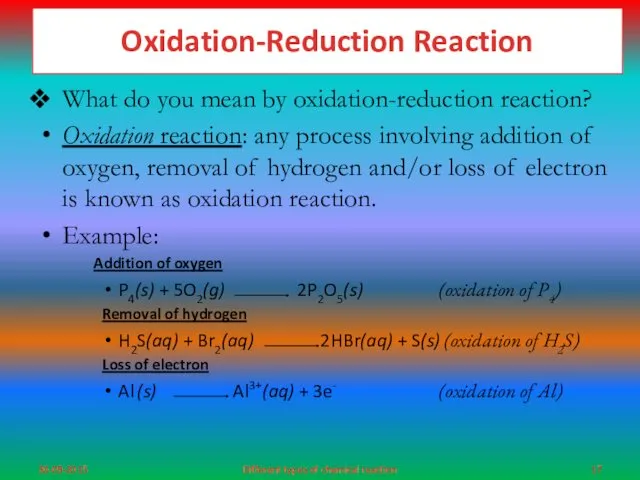
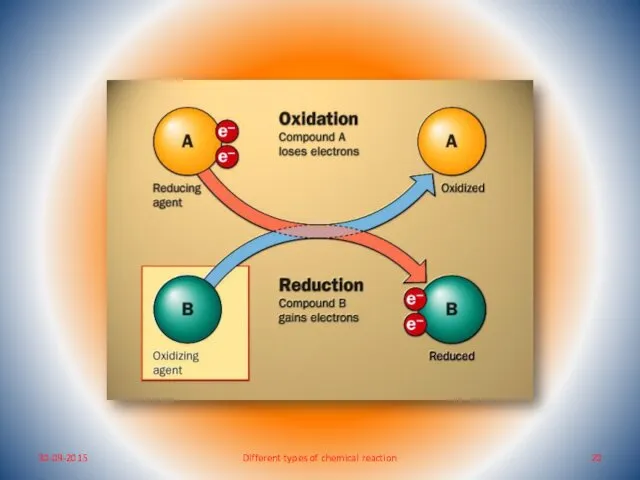
- 17. Oxidation-Reduction Reaction What do you mean by oxidation-reduction reaction? Oxidation reaction: any process involving addition of
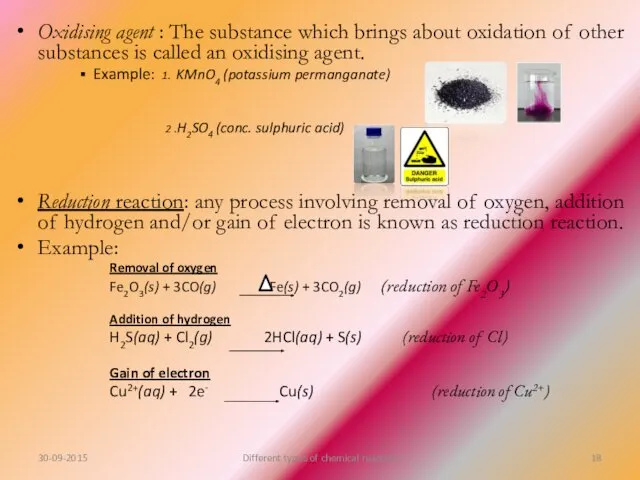
- 18. Oxidising agent : The substance which brings about oxidation of other substances is called an oxidising
- 19. Reducing agent: The substance which brings about reduction of other substance is called a reducing agent.
- 20. Different types of chemical reaction 30-09-2015
- 21. What is precipitation reaction? The reaction in which one of the products formed is an insoluble
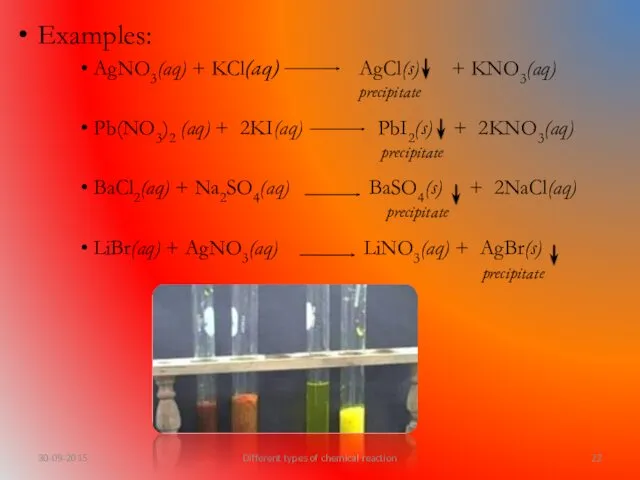
- 22. Examples: AgNO3(aq) + KCl(aq) AgCl(s) + KNO3(aq) Pb(NO3)2 (aq) + 2KI(aq) PbI2(s) + 2KNO3(aq) BaCl2(aq) +
- 23. What do you mean by exothermic and endothermic reaction? Reaction which is accompanied by evolution of
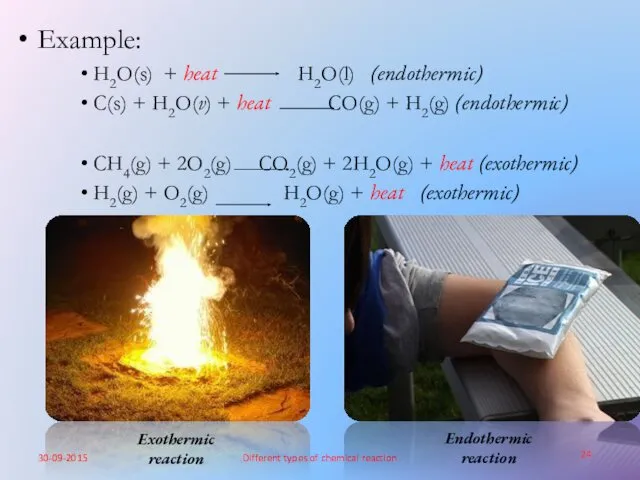
- 24. Example: H2O(s) + heat H2O(l) (endothermic) C(s) + H2O(v) + heat CO(g) + H2(g) (endothermic) CH4(g)
- 26. Скачать презентацию


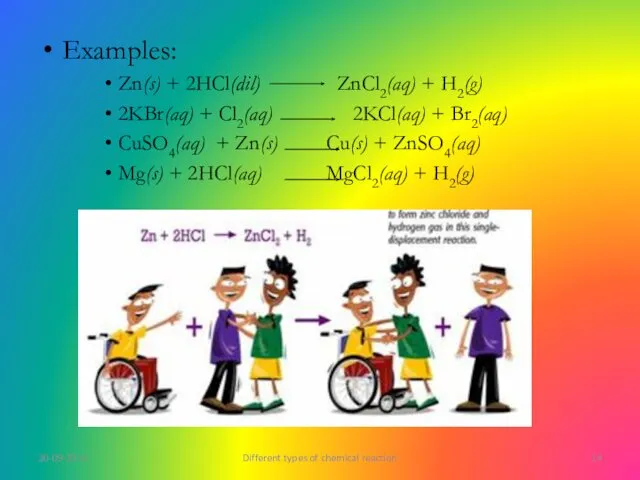

 Основные понятия и законы химии. Тема1
Основные понятия и законы химии. Тема1 Методы определения физико-химических условий минерало-и рудообразования
Методы определения физико-химических условий минерало-и рудообразования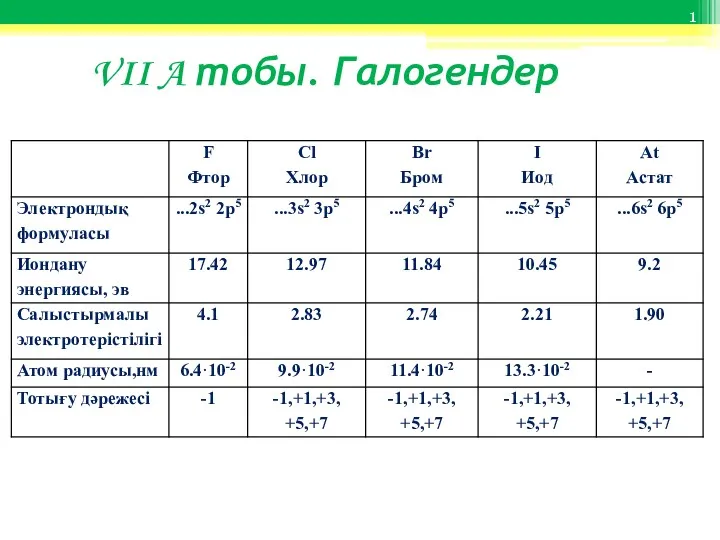 VII A тобы. Галогендер
VII A тобы. Галогендер Соединения железа Fe+2 и Fe+3
Соединения железа Fe+2 и Fe+3 Феноли (бензенол)
Феноли (бензенол) Характеристика химических элементов и химических реакций
Характеристика химических элементов и химических реакций Минерал лазурит. Месторождения
Минерал лазурит. Месторождения Неметаллы: общая характеристика. 9 класс
Неметаллы: общая характеристика. 9 класс Тема 1. Обработка вооружения, техники и обмундирования. Дегазирующие, дезактивирующие и дезинфицирующие вещества и растворы
Тема 1. Обработка вооружения, техники и обмундирования. Дегазирующие, дезактивирующие и дезинфицирующие вещества и растворы Чисті речовини та суміші. (7 клас)
Чисті речовини та суміші. (7 клас) Неметаллы. Общая характеристика
Неметаллы. Общая характеристика Предмет аналитической химии и ее основные понятия
Предмет аналитической химии и ее основные понятия Особенность, или Закономерность в строении атомов элементов. Периодическая система химических элементов Д.И. Менделеева
Особенность, или Закономерность в строении атомов элементов. Периодическая система химических элементов Д.И. Менделеева Азотная кислота. Получение, свойства. Нитраты, азотные удобрения
Азотная кислота. Получение, свойства. Нитраты, азотные удобрения Химические реакции
Химические реакции Откуда берутся кристаллы
Откуда берутся кристаллы Одноатомные спирты
Одноатомные спирты Фазовые диаграммы и статистическая термодинамика бинарных m-h систем
Фазовые диаграммы и статистическая термодинамика бинарных m-h систем Молекулярная кулинария
Молекулярная кулинария Углеводы. Моносахариды. Лекция 5
Углеводы. Моносахариды. Лекция 5 Закон постоянства состава вещества
Закон постоянства состава вещества Химическая промышленность и химическая технология
Химическая промышленность и химическая технология Окисно-відновні реакції, їхнє значення. Складання найпростіших окисно-відновних реакцій, добір коефіцієнтів
Окисно-відновні реакції, їхнє значення. Складання найпростіших окисно-відновних реакцій, добір коефіцієнтів Вода: фізичні та хімічні властивості. Поширеність в природі
Вода: фізичні та хімічні властивості. Поширеність в природі Искусственные и трансурановые элементы
Искусственные и трансурановые элементы Аллотропные модификации кремния
Аллотропные модификации кремния Периодический закон и свойства химических элементов
Периодический закон и свойства химических элементов Химическая связь
Химическая связь