Содержание
- 2. After completing this topic, you should be able to: Describe the importance of carbon to life’s
- 3. © 2016 Pearson Education, Ltd. Introduction to Organic Compounds Properties of carbon Functional groups Cells make/break
- 4. Life’s molecular diversity is based on the properties of carbon Almost all the molecules a cell
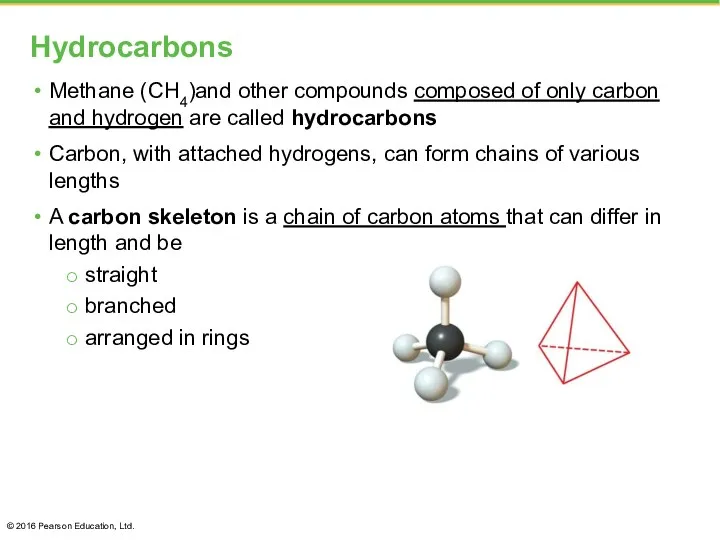
- 5. Hydrocarbons Methane (CH4)and other compounds composed of only carbon and hydrogen are called hydrocarbons Carbon, with
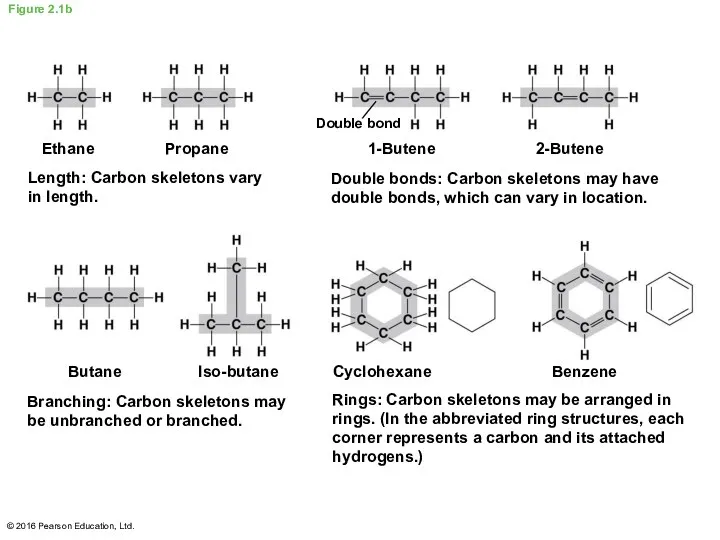
- 6. Figure 2.1b Butane Length: Carbon skeletons vary in length. Propane Double bonds: Carbon skeletons may have
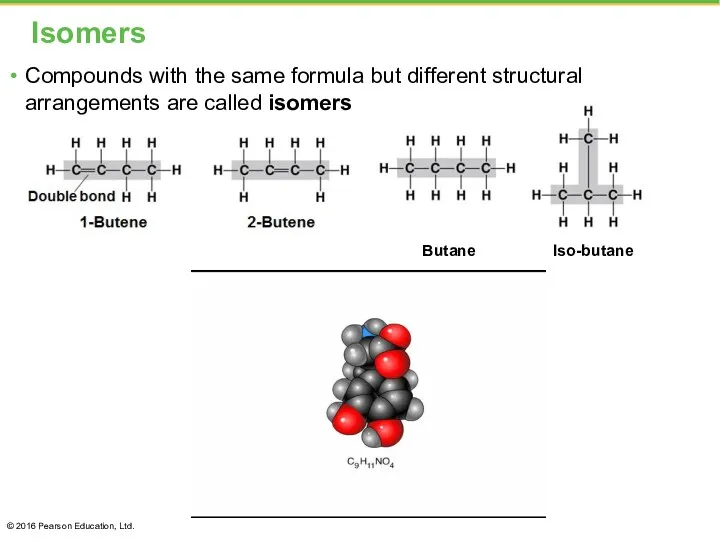
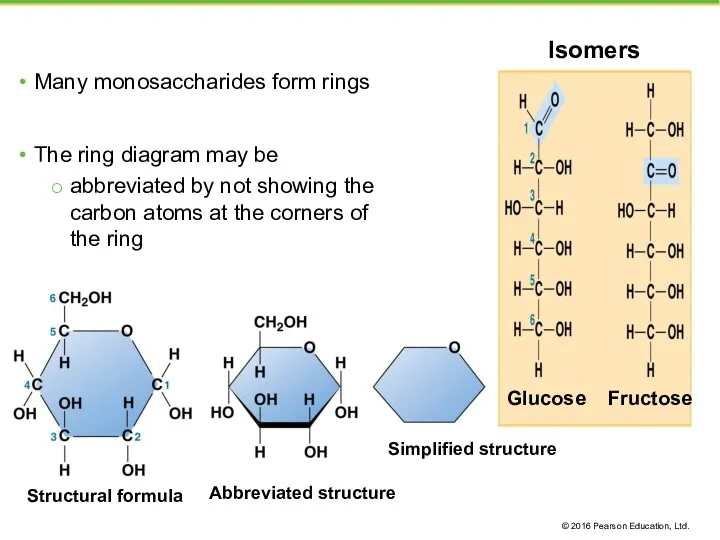
- 7. Isomers Compounds with the same formula but different structural arrangements are called isomers © 2016 Pearson
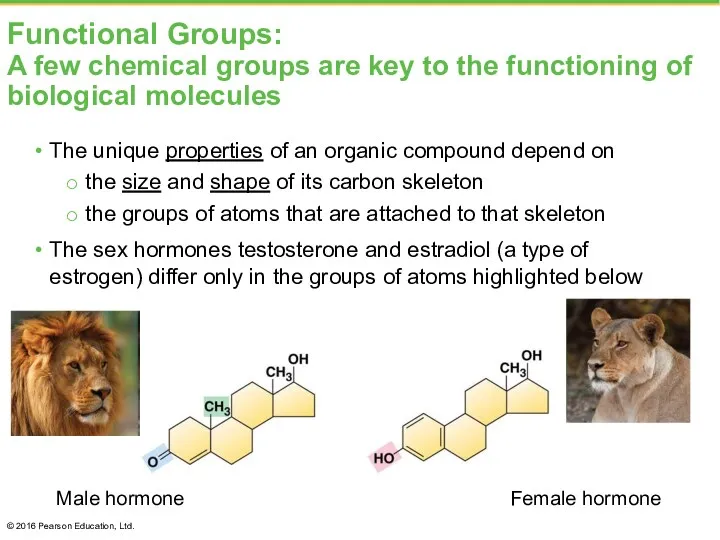
- 8. Functional Groups: A few chemical groups are key to the functioning of biological molecules The unique
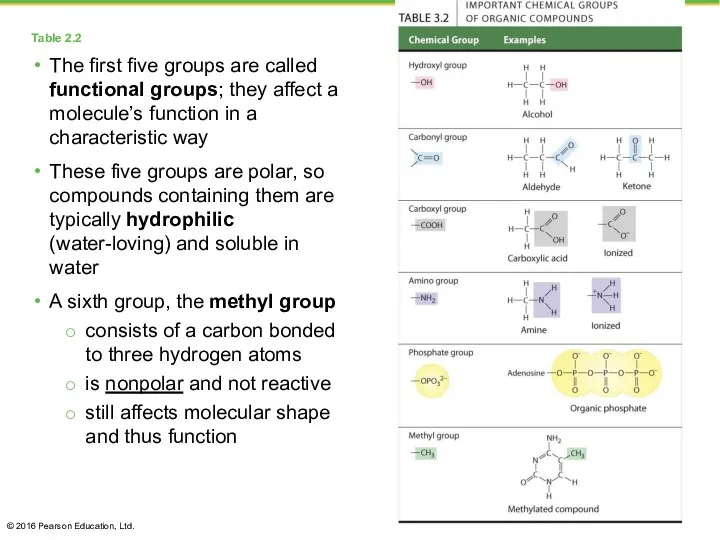
- 9. Table 2.2 The first five groups are called functional groups; they affect a molecule’s function in
- 10. Cells make large molecules from a limited set of small molecules There are four classes of
- 11. The four classes of biological molecules contain very large molecules They are often called macromolecules because
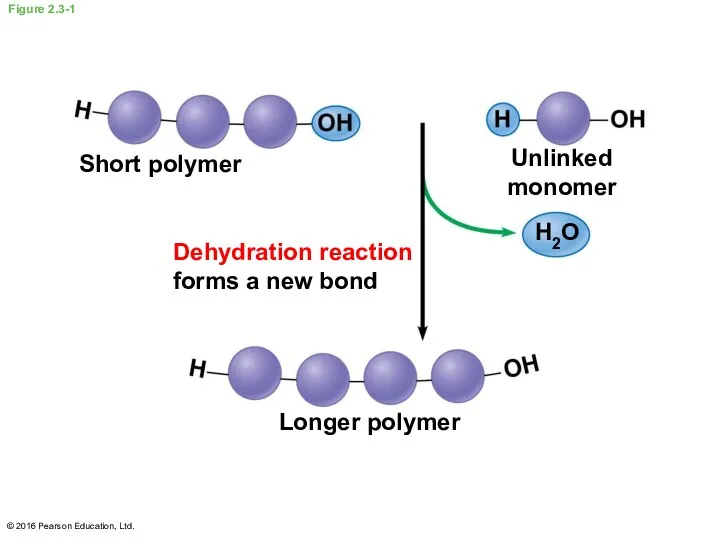
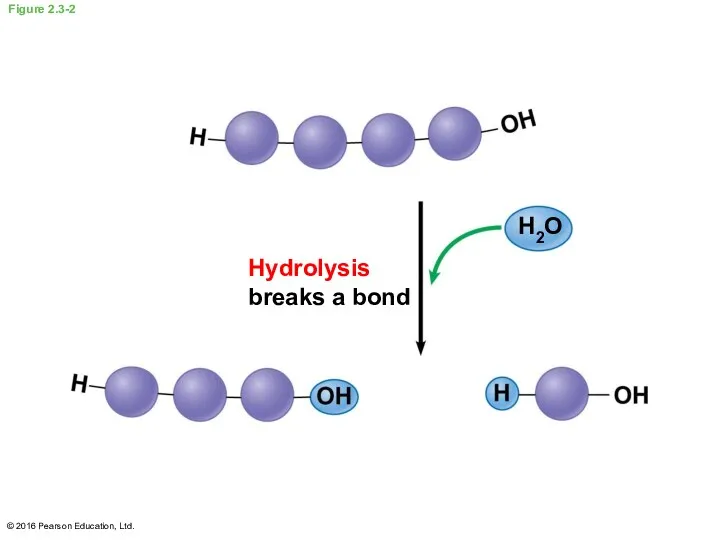
- 12. Dehydration and Hydrolysis Monomers are linked together to form polymers through dehydration reactions, which remove water
- 13. Figure 2.3-1 Short polymer Unlinked monomer Dehydration reaction forms a new bond H2O Longer polymer ©
- 14. Figure 2.3-2 Hydrolysis breaks a bond H2O © 2016 Pearson Education, Ltd.
- 15. © 2016 Pearson Education, Ltd. Carbohydrates Monosaccharide Disaccharide Polysaccharide
- 16. Monosaccharides: the simplest carbohydrates Carbohydrates range from small sugar molecules (monomers) to large polysaccharides Sugar monomers
- 17. Monosaccharides can be hooked together by dehydration reactions to form more complex sugars Polysaccharides The carbon
- 18. Many monosaccharides form rings The ring diagram may be abbreviated by not showing the carbon atoms
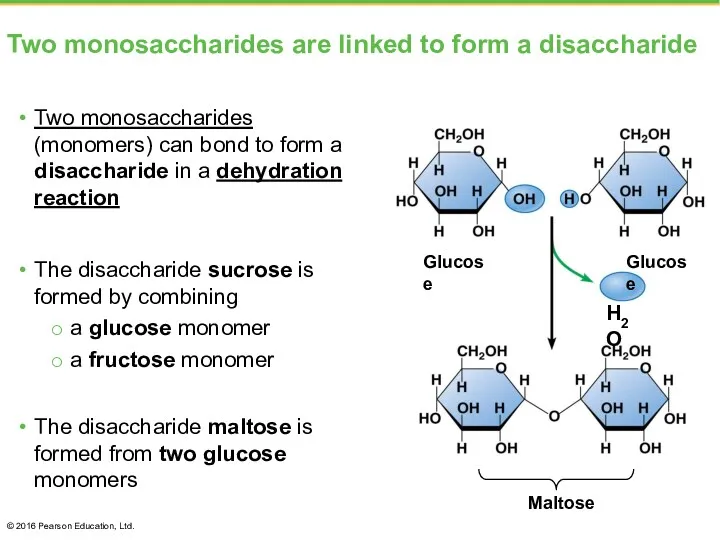
- 19. Two monosaccharides are linked to form a disaccharide Two monosaccharides (monomers) can bond to form a
- 20. Polysaccharides: Polysaccharides are macromolecules, polymers composed of thousands of monosaccharides Polysaccharides may function as storage molecules
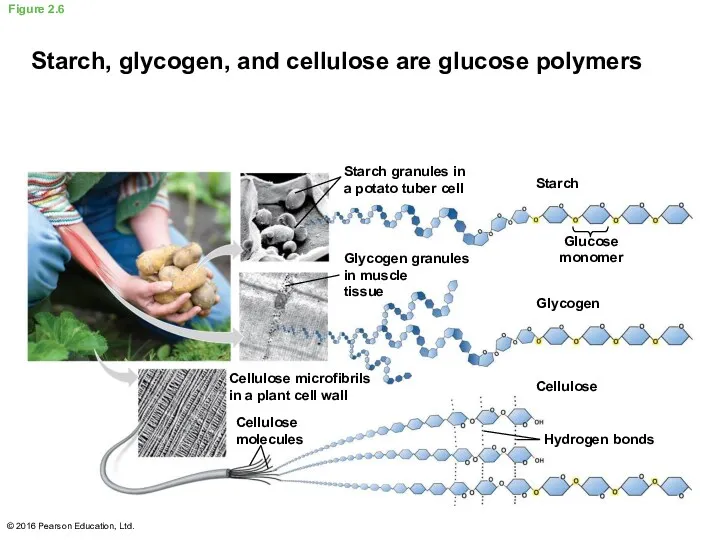
- 21. Polysaccharides are long chains of sugar units Starch is composed of glucose monomers used by plants
- 22. Figure 2.6 © 2016 Pearson Education, Ltd. Starch, glycogen, and cellulose are glucose polymers
- 23. © 2016 Pearson Education, Ltd. Lipids Fats Phospholipids Steroids
- 24. Lipids are water insoluble (hydrophobic, or water-fearing) compounds are important in long-term energy storage contain twice
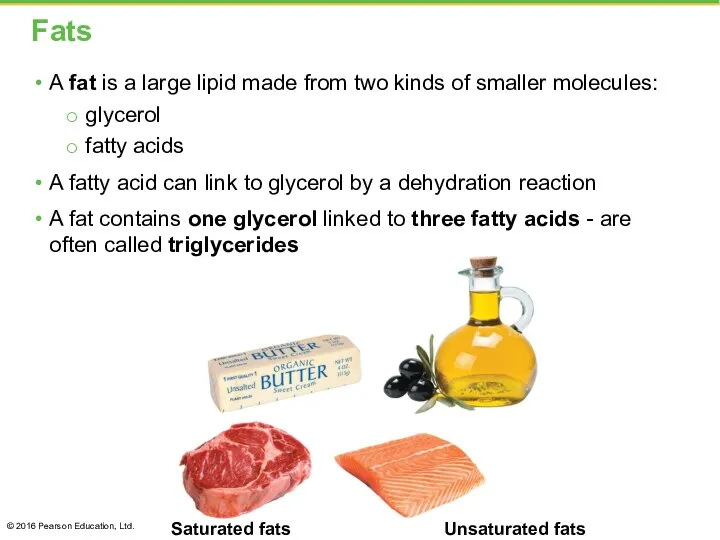
- 25. Fats A fat is a large lipid made from two kinds of smaller molecules: glycerol fatty
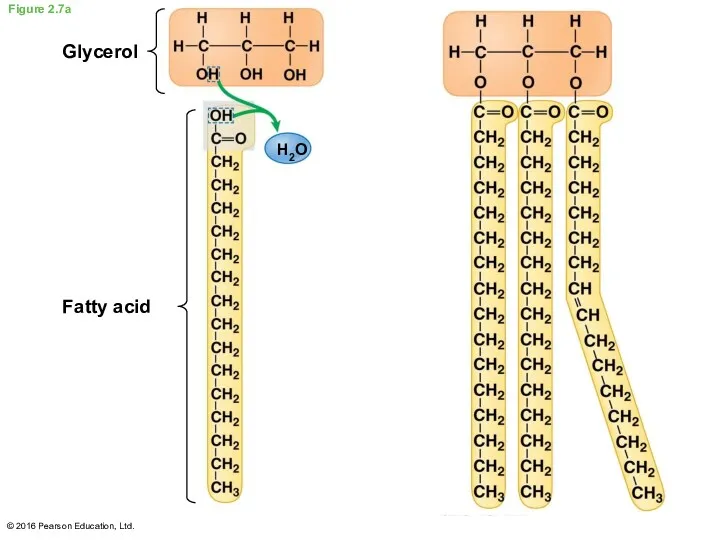
- 26. Figure 2.7a Glycerol Fatty acid H2O © 2016 Pearson Education, Ltd.
- 27. Fats are lipids that are mostly energy-storage molecules Some fatty acids contain one or more double
- 28. Unsaturated fats are referred to as oils Most animal fats are saturated fats Hydrogenated vegetable oils
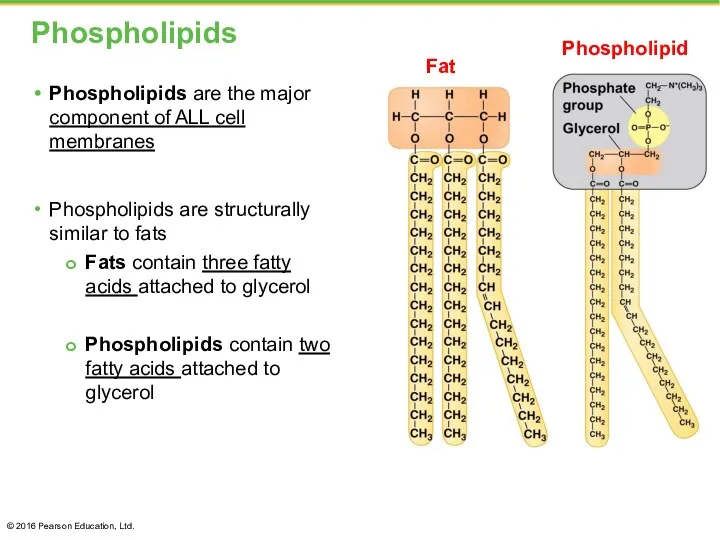
- 29. Phospholipids Phospholipids are the major component of ALL cell membranes Phospholipids are structurally similar to fats
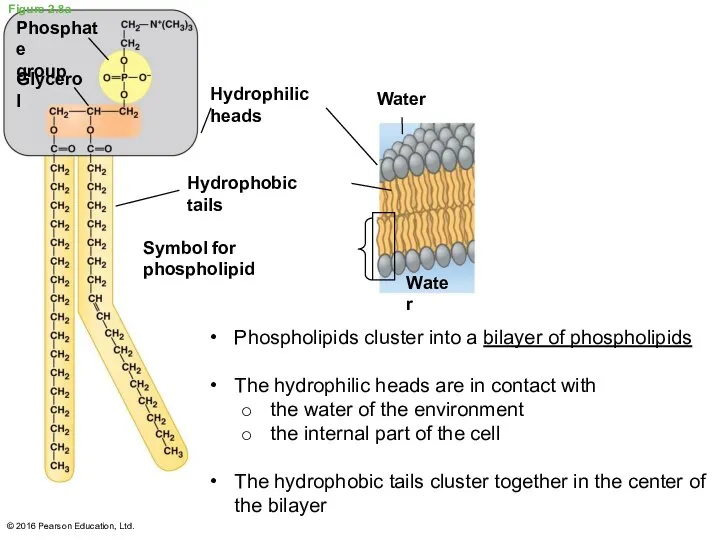
- 30. Figure 2.8a © 2016 Pearson Education, Ltd. Phospholipids cluster into a bilayer of phospholipids The hydrophilic
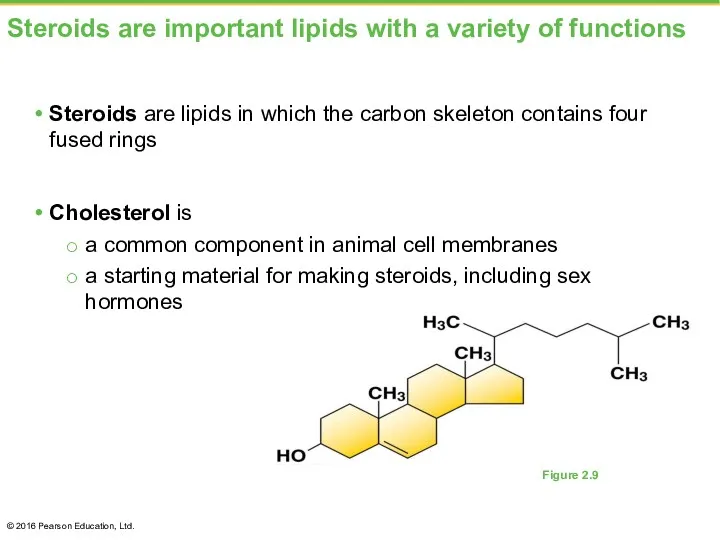
- 31. Steroids are important lipids with a variety of functions Steroids are lipids in which the carbon
- 32. Proteins © 2016 Pearson Education, Ltd.
- 33. Proteins Proteins are involved in nearly every dynamic function in your body very diverse, with tens
- 34. Types of Proteins Besides enzymes, other types of proteins include transport proteins embedded in cell membranes,
- 35. The functions of different types of proteins depend on their individual shapes The shape of a
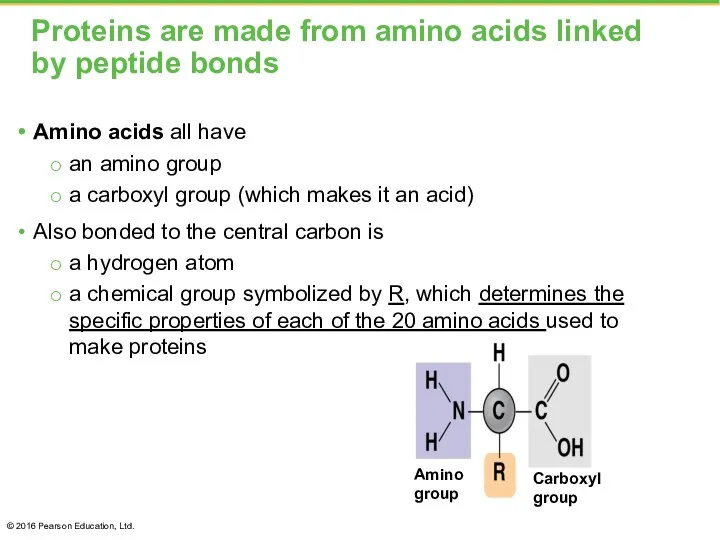
- 36. Proteins are made from amino acids linked by peptide bonds Amino acids all have an amino
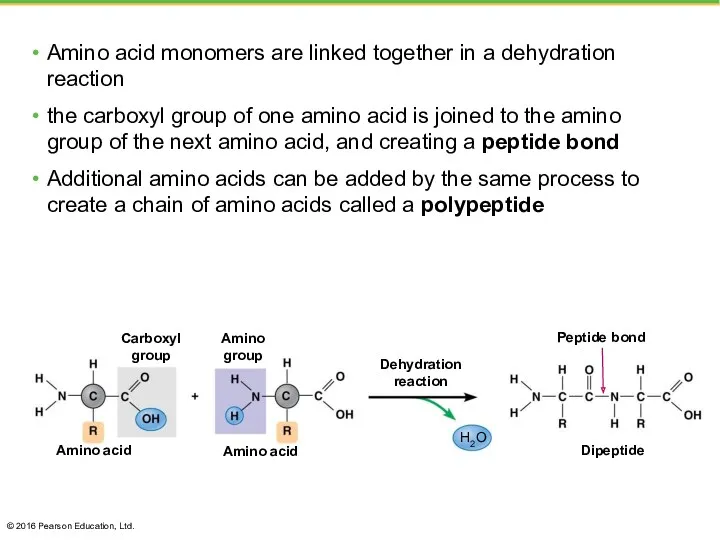
- 37. Amino acid monomers are linked together in a dehydration reaction the carboxyl group of one amino
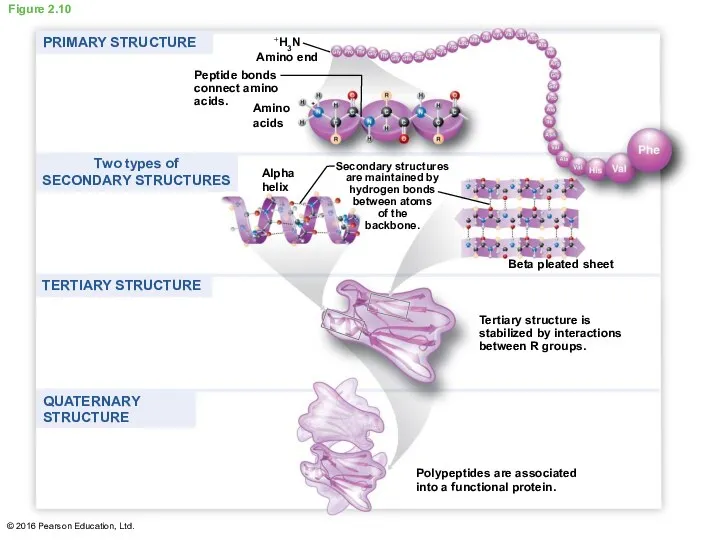
- 38. A protein’s functional shape results from four levels of structure A protein can have four levels
- 39. Figure 2.10 Amino acids +H3N Amino end Peptide bonds connect amino acids. Alpha helix Secondary structures
- 40. Nucleic Acids © 2016 Pearson Education, Ltd.
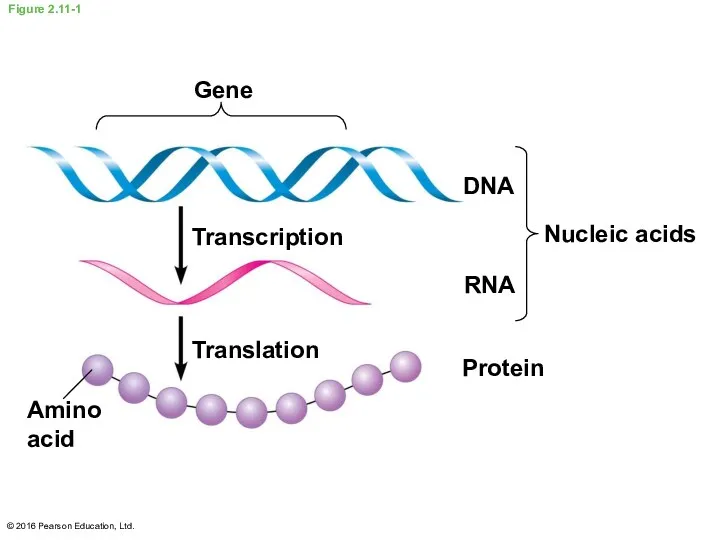
- 41. DNA and RNA are the two types of nucleic acids The amino acid sequence of a
- 42. Figure 2.11-1 Gene Transcription Translation Amino acid DNA RNA Protein Nucleic acids © 2016 Pearson Education,
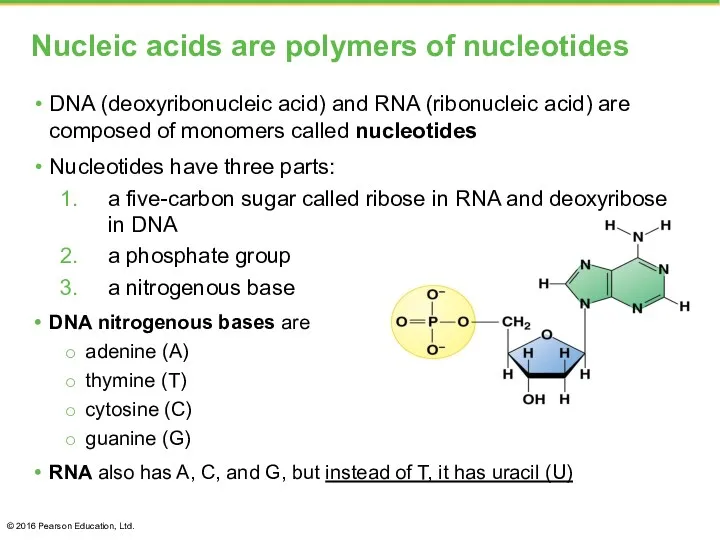
- 43. Nucleic acids are polymers of nucleotides DNA (deoxyribonucleic acid) and RNA (ribonucleic acid) are composed of
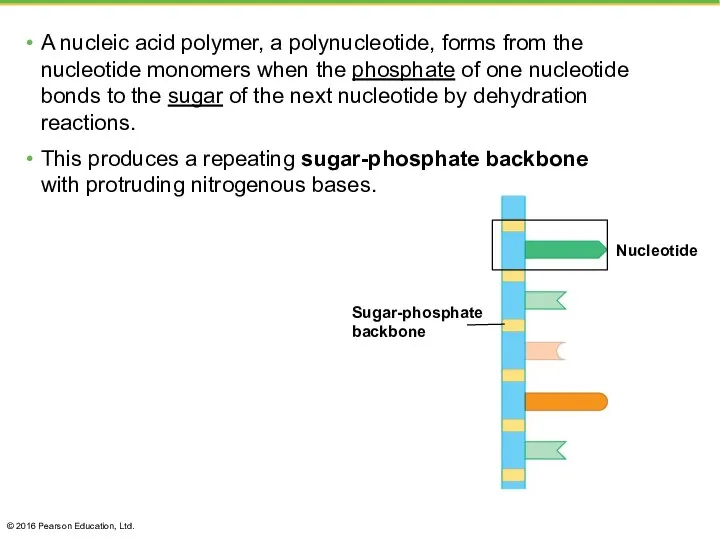
- 44. A nucleic acid polymer, a polynucleotide, forms from the nucleotide monomers when the phosphate of one
- 46. Скачать презентацию









 Материаловедение. Свойства материалов. (Тема 2)
Материаловедение. Свойства материалов. (Тема 2) Электрометрические методы анализа ЛВ. Термографические методы
Электрометрические методы анализа ЛВ. Термографические методы Темір. Жай заттармен
Темір. Жай заттармен Игра по химии по теме Атомы химических элементов. Простые вещества
Игра по химии по теме Атомы химических элементов. Простые вещества Полимеры. Структура и свойства
Полимеры. Структура и свойства Карбонильные соединения
Карбонильные соединения Магматизм. (Лекция 6)
Магматизм. (Лекция 6) Гидролиз солей
Гидролиз солей Углеводы
Углеводы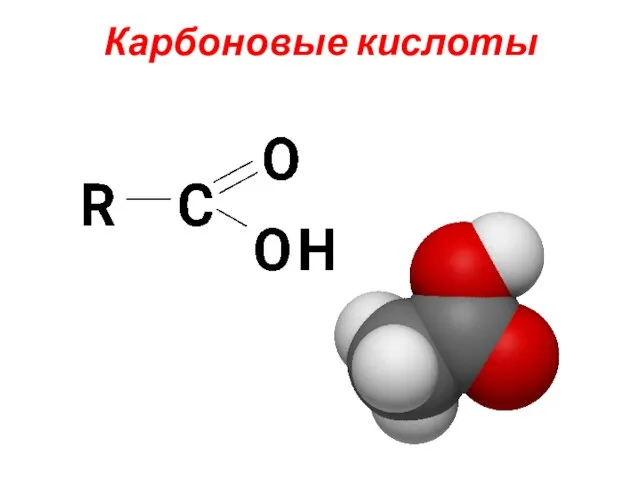 Карбоновые кислоты. Классификация карбоновых кислот
Карбоновые кислоты. Классификация карбоновых кислот Высокомолекулярные соединения
Высокомолекулярные соединения Каталитические процессы нефтепереработки
Каталитические процессы нефтепереработки Химические формулы
Химические формулы Химический анализ состава йогуртов наиболее популярных торговых марок
Химический анализ состава йогуртов наиболее популярных торговых марок Минералы. Химическая классификация
Минералы. Химическая классификация Закон Авогадро. Молярный объём газов
Закон Авогадро. Молярный объём газов Оксидтер
Оксидтер Состав веществ. Причины многообразия веществ
Состав веществ. Причины многообразия веществ Признаки и условия протекания химических реакций
Признаки и условия протекания химических реакций Электролитическая диссоциация. 9 класс
Электролитическая диссоциация. 9 класс Реакции ионного обмена
Реакции ионного обмена Типы химических реакций. Практическая работа
Типы химических реакций. Практическая работа Почему мыло пенится
Почему мыло пенится 20230419_alkiny_uglub
20230419_alkiny_uglub Основные понятия органической химии
Основные понятия органической химии Фосфор и его соединения
Фосфор и его соединения Кaрбоновые кислоты
Кaрбоновые кислоты Неметаллы: общая характеристика. 9 класс
Неметаллы: общая характеристика. 9 класс