Содержание
- 2. LESSON OBJECTIVES: Water and its structure To explore the unique properties of water as the cohesion,
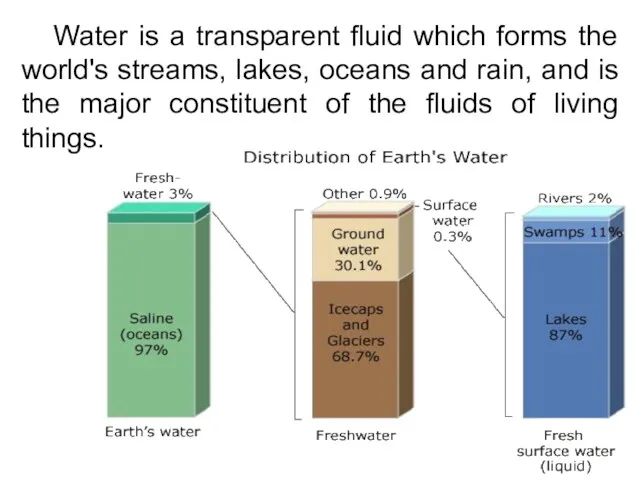
- 3. Water is a transparent fluid which forms the world's streams, lakes, oceans and rain, and is
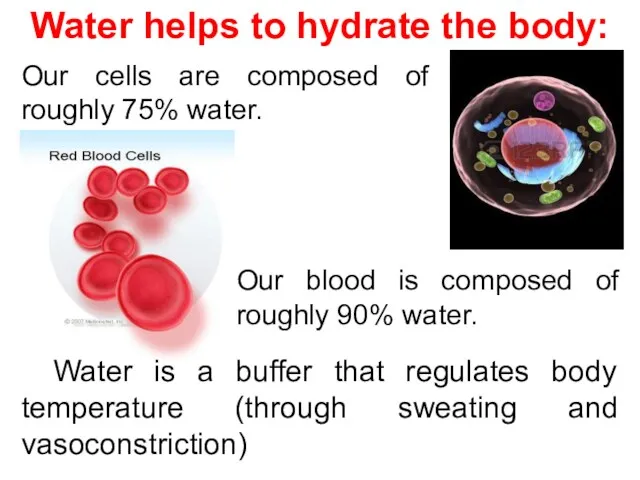
- 4. Water is a buffer that regulates body temperature (through sweating and vasoconstriction) Water helps to hydrate
- 5. Water is a liquid at standard ambient temperature and pressure, but it often co-exists on Earth
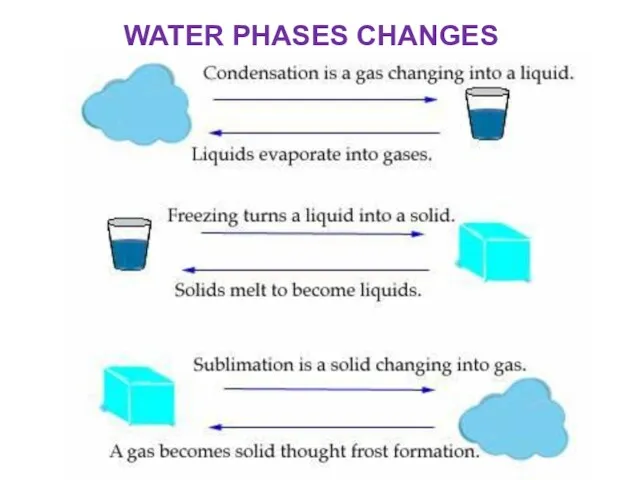
- 6. WATER PHASES CHANGES
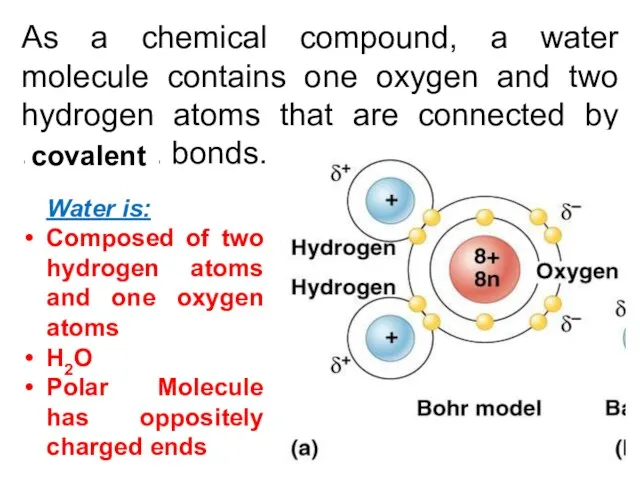
- 7. As a chemical compound, a water molecule contains one oxygen and two hydrogen atoms that are
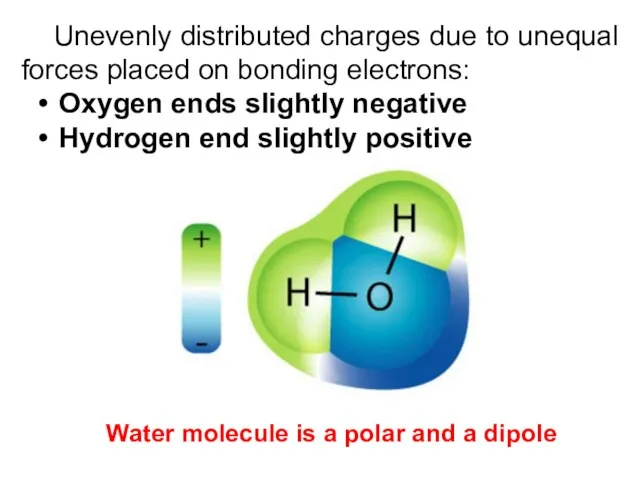
- 8. Unevenly distributed charges due to unequal forces placed on bonding electrons: Oxygen ends slightly negative Hydrogen
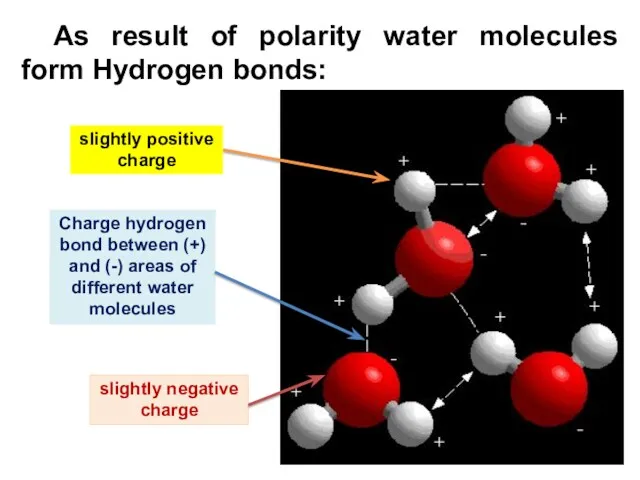
- 9. As result of polarity water molecules form Hydrogen bonds: slightly positive charge Charge hydrogen bond between
- 10. PHYSICAL PROPERTIES OF WATER: Water has a high specific heat. Water in a pure state has
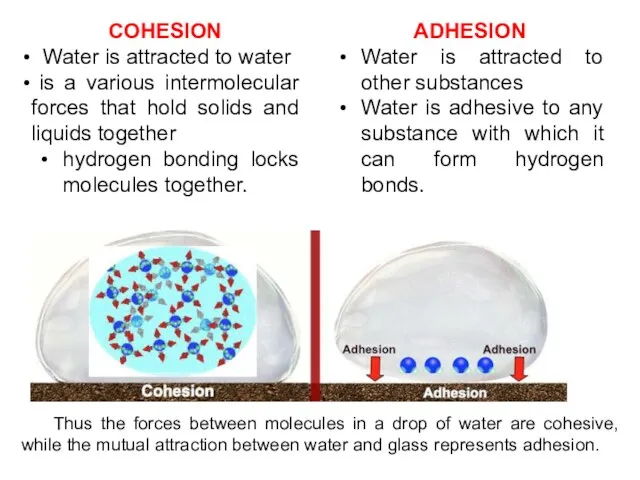
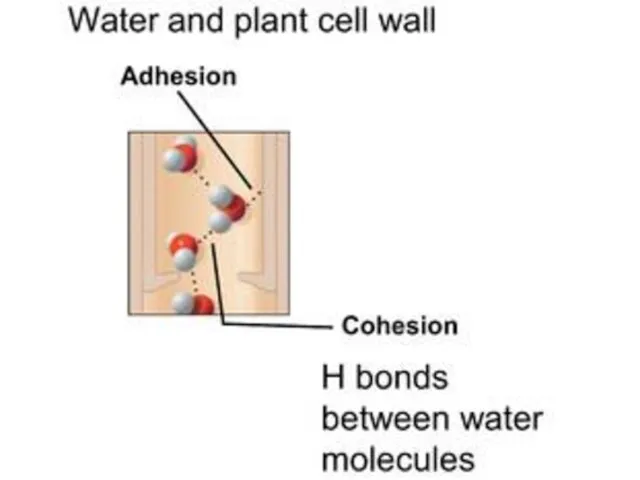
- 11. COHESION Water is attracted to water is a various intermolecular forces that hold solids and liquids
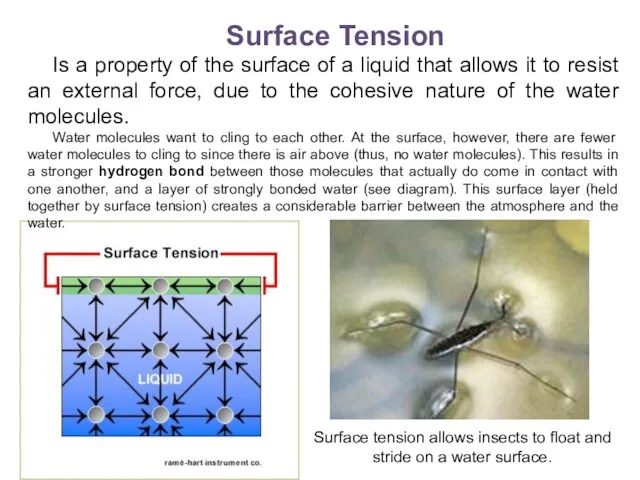
- 13. Surface Tension Is a property of the surface of a liquid that allows it to resist
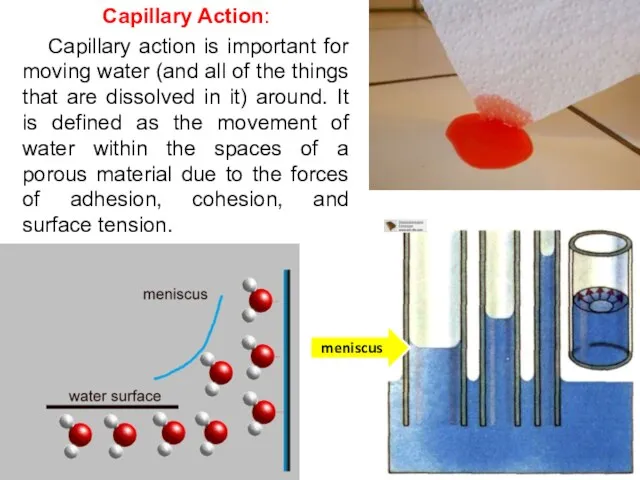
- 14. Capillary Action: Capillary action is important for moving water (and all of the things that are
- 15. Density: Water has a density of 1g/mL at 4 °C Water is the one of the
- 16. Properties of Water At sea level, pure water boils at 100 °C and freezes at 0
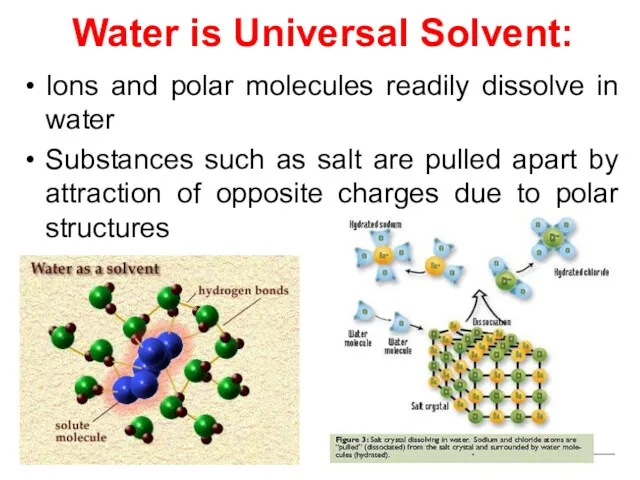
- 17. Water is Universal Solvent: Ions and polar molecules readily dissolve in water Substances such as salt
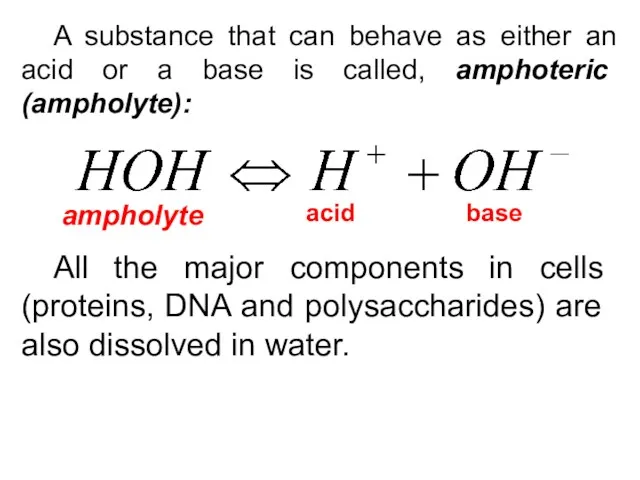
- 18. A substance that can behave as either an acid or a base is called, amphoteric (ampholyte):
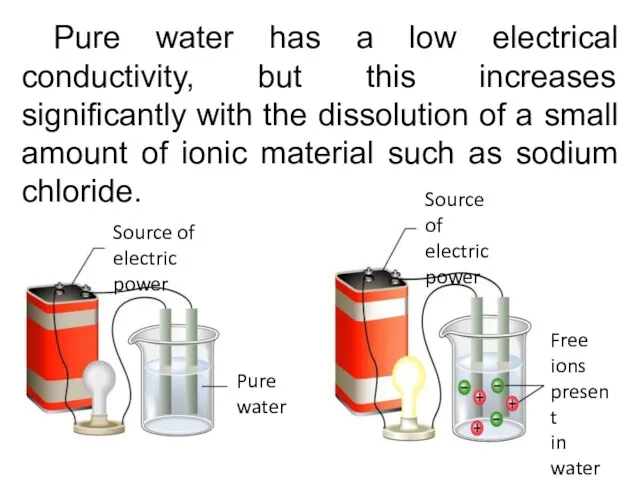
- 19. Pure water has a low electrical conductivity, but this increases significantly with the dissolution of a
- 20. CHEMICAL PROPERTIES OF WATER: pH (activity acidity) Total Acidity Alkalinity Total Hardness Chemical reactivity: water can
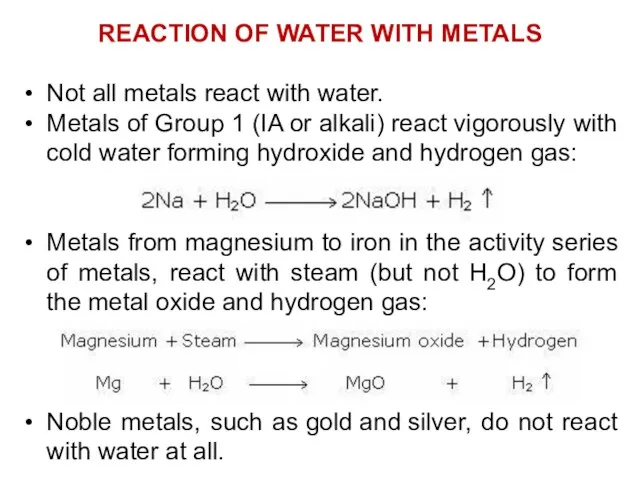
- 21. REACTION OF WATER WITH METALS Not all metals react with water. Metals of Group 1 (IA
- 22. DISSOLVING ELECTROLYTES IN WATER Solid electrolytes are composed of ions which are held together by electrostatic
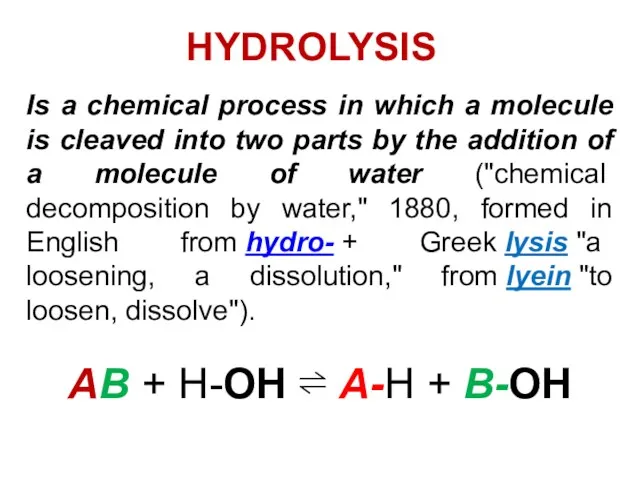
- 23. Is a chemical process in which a molecule is cleaved into two parts by the addition
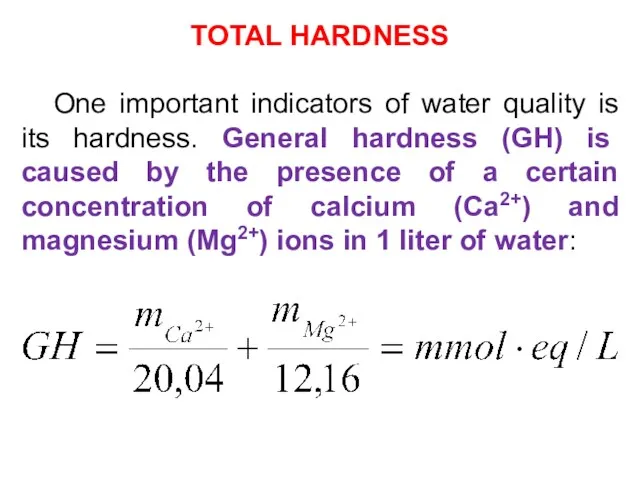
- 24. TOTAL HARDNESS One important indicators of water quality is its hardness. General hardness (GH) is caused
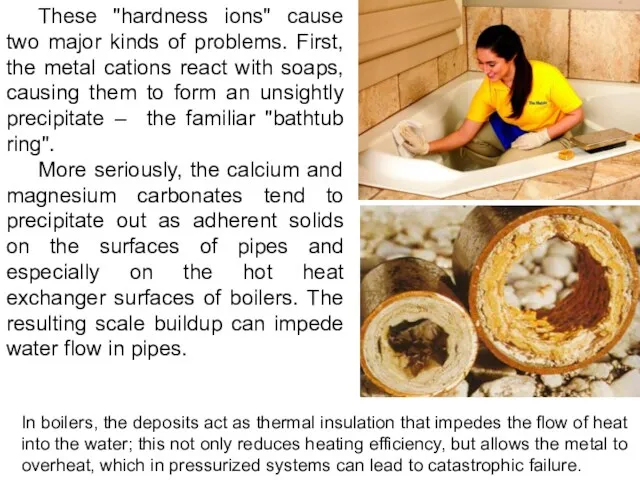
- 25. These "hardness ions" cause two major kinds of problems. First, the metal cations react with soaps,
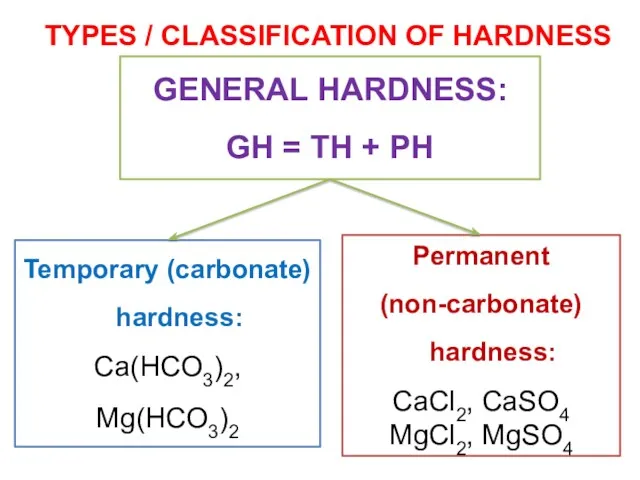
- 26. TYPES / CLASSIFICATION OF HARDNESS Temporary (carbonate) hardness: Ca(HCO3)2, Mg(HCO3)2 Permanent (non-carbonate) hardness: CaCl2, CaSO4 MgCl2,
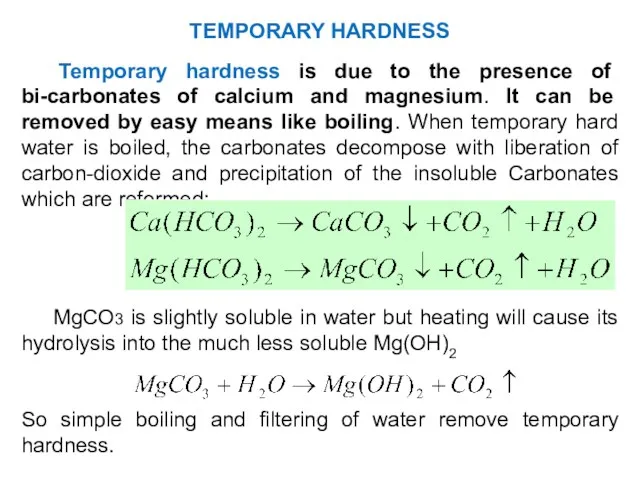
- 27. Temporary hardness is due to the presence of bi-carbonates of calcium and magnesium. It can be
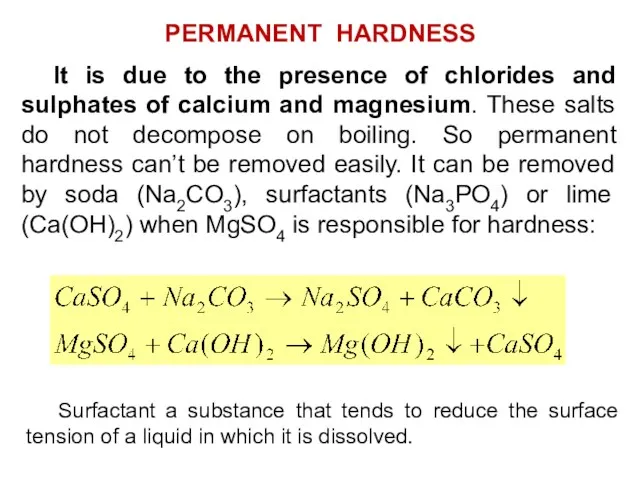
- 28. It is due to the presence of chlorides and sulphates of calcium and magnesium. These salts
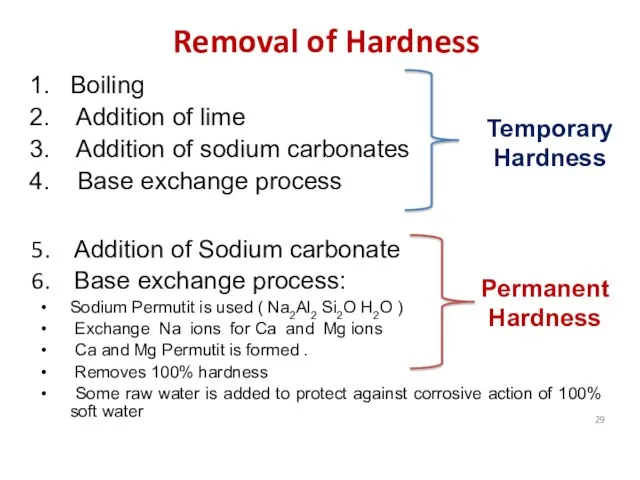
- 29. Removal of Hardness Boiling Addition of lime Addition of sodium carbonates Base exchange process Addition of
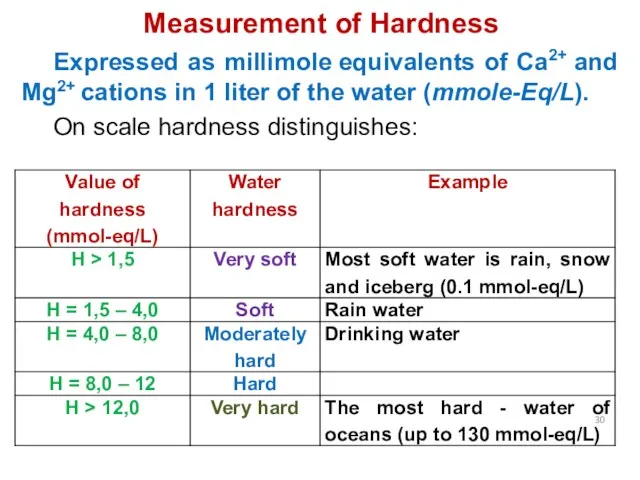
- 30. Measurement of Hardness Expressed as millimole equivalents of Ca2+ and Mg2+ cations in 1 liter of
- 31. ESTIMATION OF WATER HARDNESS Water hardness can be determined by the following 2 analytical methods: By
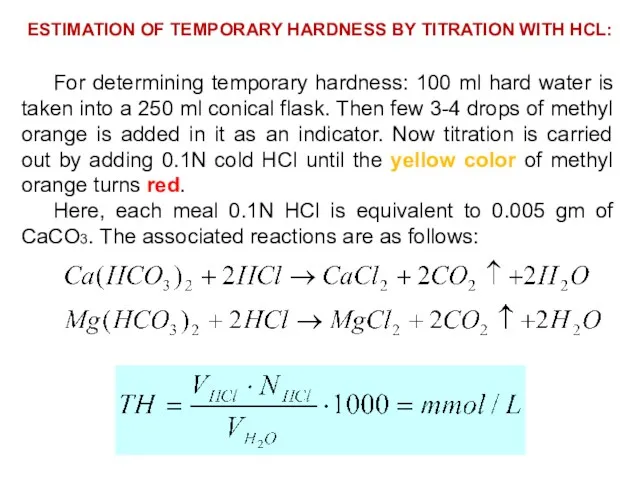
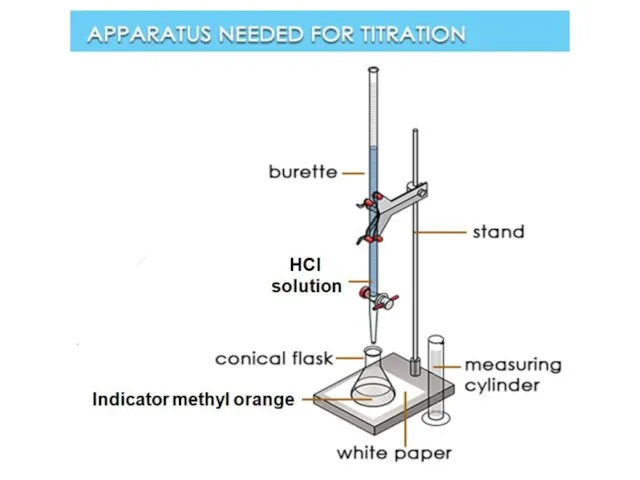
- 32. ESTIMATION OF TEMPORARY HARDNESS BY TITRATION WITH HCL: For determining temporary hardness: 100 ml hard water
- 33. Add 1ml of buffer solution (NH4OH+NH4Cl) to 100 ml of the original water sample. Add 3-4
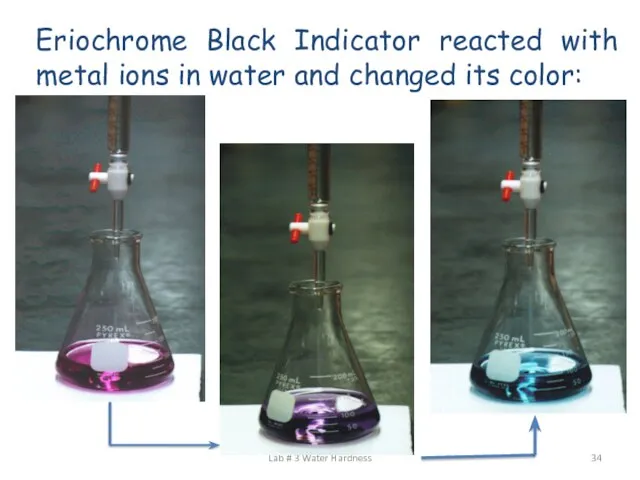
- 34. Eriochrome Black Indicator reacted with metal ions in water and changed its color: Lab # 3
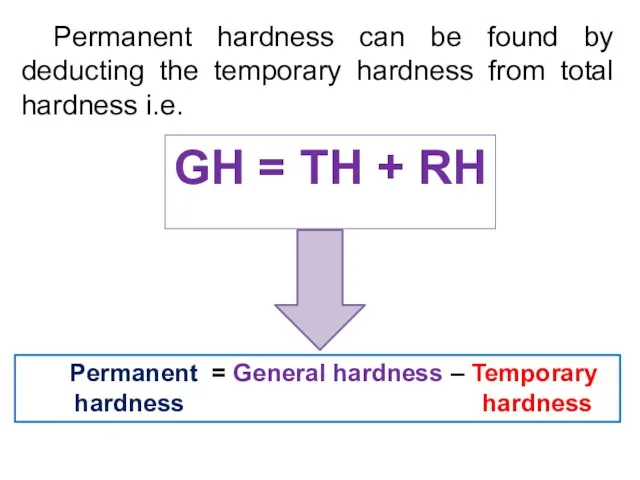
- 35. Permanent hardness can be found by deducting the temporary hardness from total hardness i.e. Permanent =
- 36. CONCLUSION: The safe drinking water is recognized water: with pH of 7 to 7.5 mmol /
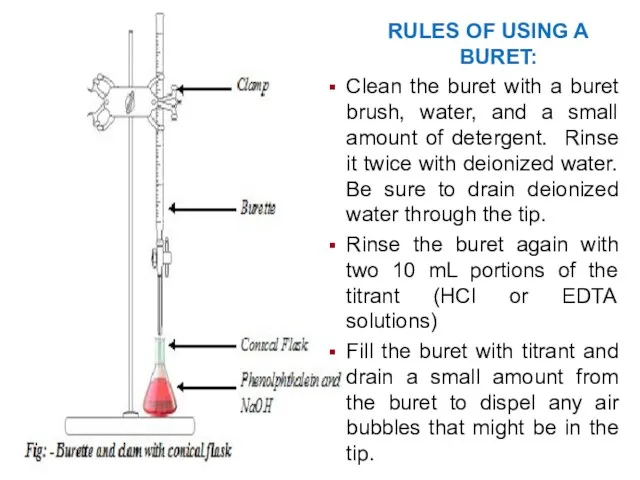
- 38. RULES OF USING A BURET: Clean the buret with a buret brush, water, and a small
- 39. Use a marker pen to create a black area on a white note card. This will
- 40. If you need to dispense less than a full drop of titrant, open the stopcock slightly
- 41. КИПЯЧЕНИЕ ВОДЫ Жесткость снижается на 30 - 40%.
- 42. ВЫМОРАЖИВАНИЕ ВОДЫ Общая жесткость снижается на 70-80%.
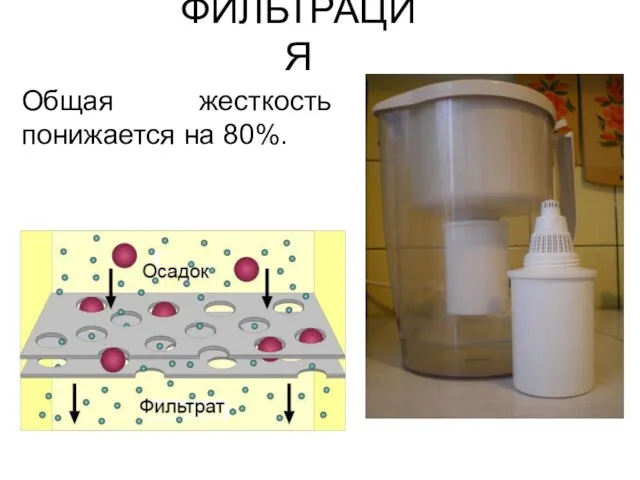
- 43. ФИЛЬТРАЦИЯ Общая жесткость понижается на 80%.
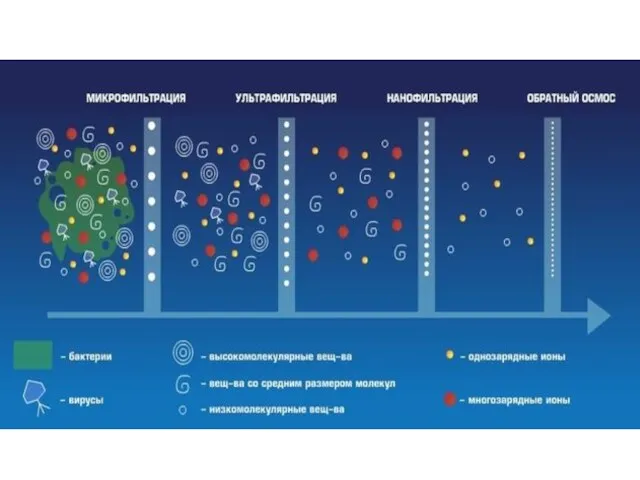
- 45. Что такое бытовой фильтр? Внутри картриджа фильтра содержится смесь из активированного угля (черные частицы) и катионообменники
- 46. УМЯГЧИТЕЛИ ВОДЫ
- 48. Скачать презентацию















 Минералы свинца
Минералы свинца Особенности химического состава клетки
Особенности химического состава клетки Товары из пластмасс. Система маркировки пластика
Товары из пластмасс. Система маркировки пластика Алюминий и его соединения. Строение атома
Алюминий и его соединения. Строение атома СПЛАВЫ. КОРРОЗИЯ МЕТАЛЛОВ
СПЛАВЫ. КОРРОЗИЯ МЕТАЛЛОВ Кислоты: классификация и химические свойства
Кислоты: классификация и химические свойства Кислородные соединения серы. 2 часть
Кислородные соединения серы. 2 часть Кінетика хімічних реакцій і хімічна рівновага
Кінетика хімічних реакцій і хімічна рівновага Магнитные наночастицы: проблемы и достижения
Магнитные наночастицы: проблемы и достижения Ионные уравнения реакций
Ионные уравнения реакций Особенности строения, реакционной способности и методы синтеза гидроксилсодержащих соединений
Особенности строения, реакционной способности и методы синтеза гидроксилсодержащих соединений Практикум по органической и биоорганической химии
Практикум по органической и биоорганической химии Химические формулы
Химические формулы Значення води і водних розчинів у природі та житті людини. Кислотні дощі
Значення води і водних розчинів у природі та житті людини. Кислотні дощі БАЗ синтездеудің химиялық технологиясы Синтетикалық түсінік
БАЗ синтездеудің химиялық технологиясы Синтетикалық түсінік Автомобильные пластичные смазки
Автомобильные пластичные смазки Приготовление растворов солей с определенной массовой долей растворенного вещества
Приготовление растворов солей с определенной массовой долей растворенного вещества Азотная кислота и её соли
Азотная кислота и её соли Неорганические строительные материалы. Стекло
Неорганические строительные материалы. Стекло Химический состав клетки. Вода
Химический состав клетки. Вода Реакции ионного обмена
Реакции ионного обмена Углеводы. Общая формула углеводов Сn(H2O)m
Углеводы. Общая формула углеводов Сn(H2O)m Силикатная промышленность. 9 класс
Силикатная промышленность. 9 класс Предмет біоорганічної хімії. Класифікація, номенклатура, електронні уявлення, будова, реакційна здатність органічних сполук
Предмет біоорганічної хімії. Класифікація, номенклатура, електронні уявлення, будова, реакційна здатність органічних сполук Ауыр металлдар
Ауыр металлдар Молекулы и атомы. Броуновское движение
Молекулы и атомы. Броуновское движение Химиялық кинетика және химиялық тепе-теңдік
Химиялық кинетика және химиялық тепе-теңдік Окислительно-восстановительные реакции
Окислительно-восстановительные реакции