Thermoplastics
There are a wide range of thermoplastics, some that are rigid
and some that are extremely flexible.
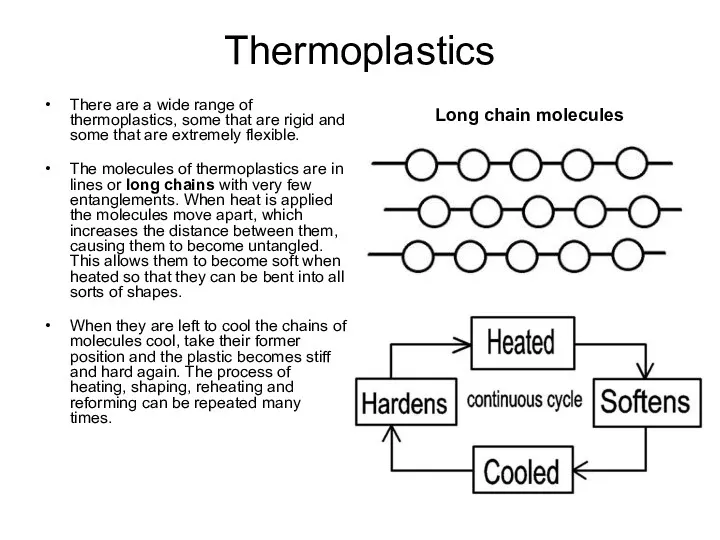
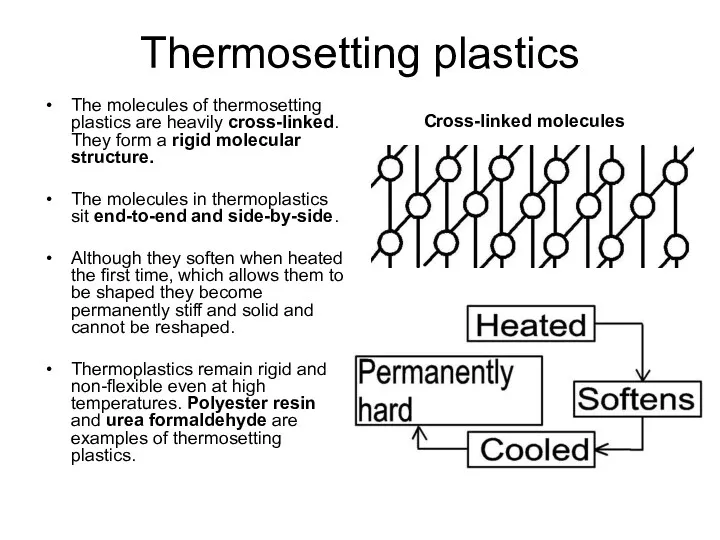
The molecules of thermoplastics are in lines or long chains with very few entanglements. When heat is applied the molecules move apart, which increases the distance between them, causing them to become untangled. This allows them to become soft when heated so that they can be bent into all sorts of shapes.
When they are left to cool the chains of molecules cool, take their former position and the plastic becomes stiff and hard again. The process of heating, shaping, reheating and reforming can be repeated many times.
Long chain molecules








 Углеводы. Глюкоза
Углеводы. Глюкоза Дипломная работа. Тема: Получение гальванических покрытий на основе цинка
Дипломная работа. Тема: Получение гальванических покрытий на основе цинка Физико-химические свойства белков. Количественные (колориметрические) методы определения концентрации белка
Физико-химические свойства белков. Количественные (колориметрические) методы определения концентрации белка Химические свойства. Оксиды, основания, кислоты и соли
Химические свойства. Оксиды, основания, кислоты и соли Химия и здоровье
Химия и здоровье Важнейшие реакции в органической химии
Важнейшие реакции в органической химии Межлабораторные сравнительные испытания качественных параметров нефтепродуктов
Межлабораторные сравнительные испытания качественных параметров нефтепродуктов Вода как среда и участник протекания биохимических процессов в организме
Вода как среда и участник протекания биохимических процессов в организме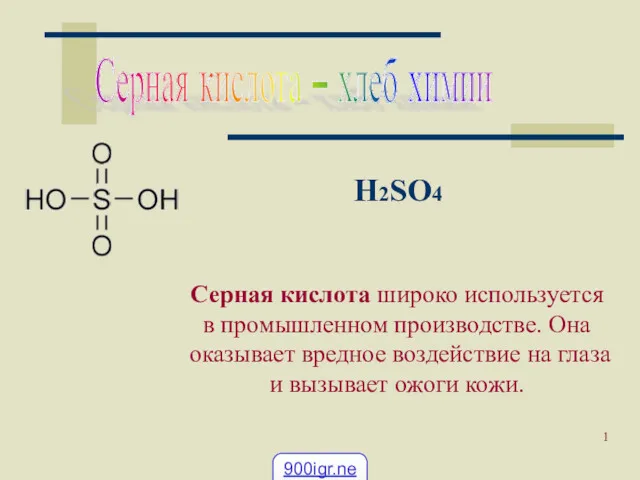 Применение серной кислоты
Применение серной кислоты Геохимическая классификация элементов
Геохимическая классификация элементов Классификация химических элементов
Классификация химических элементов Производство извести в домашних условиях. 7 класс
Производство извести в домашних условиях. 7 класс Карбонаты и гидрокарбонаты. Тест – экспресс
Карбонаты и гидрокарбонаты. Тест – экспресс Бытовая химическая грамотность
Бытовая химическая грамотность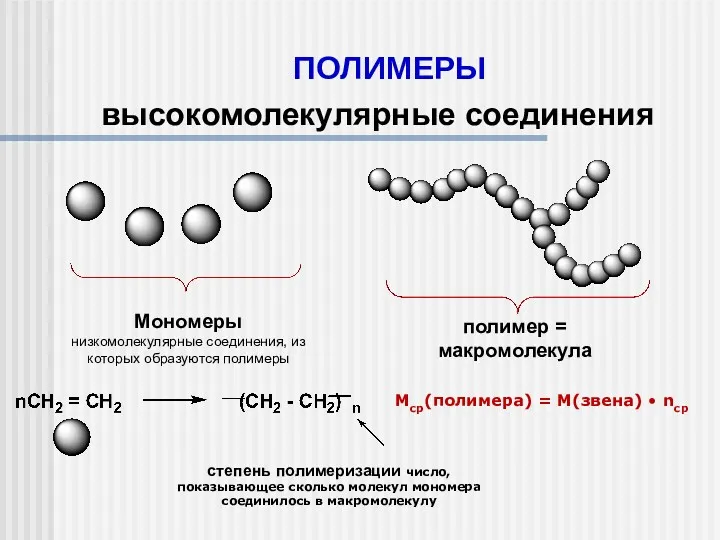 Полимеры. Высокомолекулярные соединения
Полимеры. Высокомолекулярные соединения Будова атома: ядро й електронна оболонка. Склад атомних ядер. Протонне та нуклонне число
Будова атома: ядро й електронна оболонка. Склад атомних ядер. Протонне та нуклонне число Адсорбция. Разделение однородных и неоднородных смесей
Адсорбция. Разделение однородных и неоднородных смесей Гидролиз неорганических соединений
Гидролиз неорганических соединений Получение спиртов. Применение
Получение спиртов. Применение Природный газ и нефть
Природный газ и нефть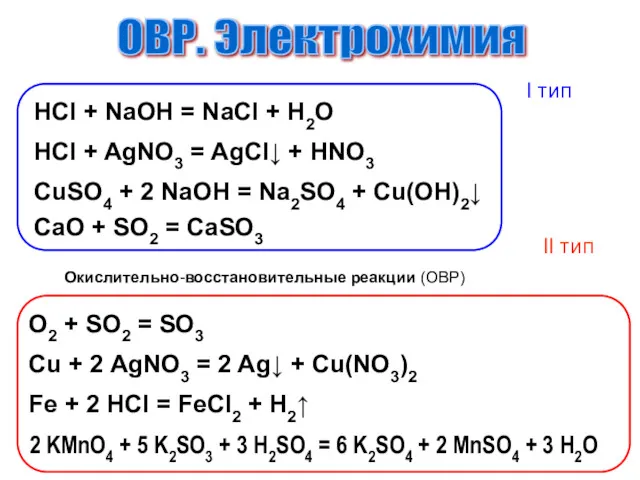 Окислительно-восстановительные процессы
Окислительно-восстановительные процессы Техника безопасности на уроках химии
Техника безопасности на уроках химии Алкани. Ізомерія та номенклатура алканів
Алкани. Ізомерія та номенклатура алканів Кислородосодержащие производные углеводородов. Спирты. Фенолы. Простые эфиры
Кислородосодержащие производные углеводородов. Спирты. Фенолы. Простые эфиры Химические реакции органических соединений
Химические реакции органических соединений Правила ДСС
Правила ДСС Кинетика химических реакций и химическое равновесие
Кинетика химических реакций и химическое равновесие Розв’язання експериментальних задач. Практична робота №2
Розв’язання експериментальних задач. Практична робота №2