Слайд 2
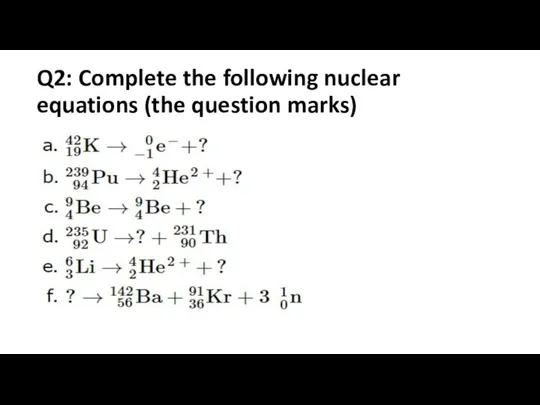
Q2: Complete the following nuclear equations (the question marks)
Слайд 3

Pre-lesson activity:
What is the atomic mass?
Why we do not use the
absolute atomic mass?
How the relative atomic mass was calculated?
What is the value of amu?
Why the atomic masses in the periodic table are not necessarily whole numbers?
Слайд 4

Theme of the lesson
Atomic mass
Слайд 5

Learning objectives
Calculate relative atomic, molecular and formula masses.
Explain why the atomic
masses in the periodic table are not necessarily whole numbers.
Calculate relative isotopic ratios from molar mass.
Слайд 6

Success criteria
Student achieves if
He/she will be able to calculate relative
atomic, molecular and formula masses
He/she can explain why the atomic masses in the periodic table are not necessarily whole numbers
He/she will be able to calculate relative isotopic ratios from molar mass
Слайд 7
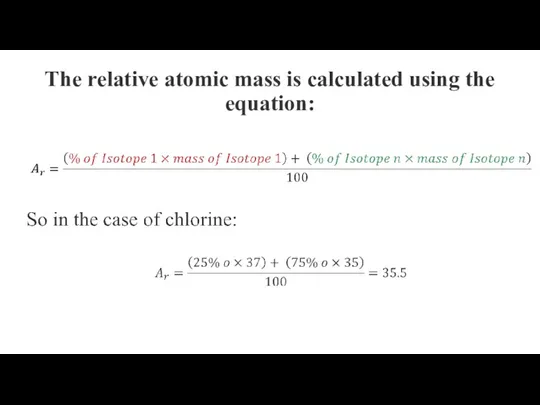
The relative atomic mass is calculated using the equation:






 Хімічні формули речовин
Хімічні формули речовин Водород
Водород Химиялық қауіптілер. Нитраттар
Химиялық қауіптілер. Нитраттар Кислоты в свете теории электролитической диссоциации (ТЭД)
Кислоты в свете теории электролитической диссоциации (ТЭД) Кристаллическое строение и свойства металлов
Кристаллическое строение и свойства металлов Материаловедение промышленного производства. Особенности строения твердых тел
Материаловедение промышленного производства. Особенности строения твердых тел Неметаллы: общая характеристика. 9 класс
Неметаллы: общая характеристика. 9 класс Понятия про синтетические лекарственные средства
Понятия про синтетические лекарственные средства Основи. Властивості, застосування гідроксидів Натрію і Калію
Основи. Властивості, застосування гідроксидів Натрію і Калію Акцепторы катионов и анионов. Краун-эфиры и близкие структурные аналоги: поданды, лариатэфиры. Супрамолекулярная фотоника
Акцепторы катионов и анионов. Краун-эфиры и близкие структурные аналоги: поданды, лариатэфиры. Супрамолекулярная фотоника Химия в Великую Отечественную войну
Химия в Великую Отечественную войну Массовая доля вещества в растворе
Массовая доля вещества в растворе Строение атома. Периодический закон и периодическая система элементов
Строение атома. Периодический закон и периодическая система элементов Современные химические технологии
Современные химические технологии Каталитический риформинг
Каталитический риформинг Получение, собирание, распознавание газов. (Практическая работа 2)
Получение, собирание, распознавание газов. (Практическая работа 2) Углеводороды. Предельные нециклические (ациклические) углеводороды. Алканы
Углеводороды. Предельные нециклические (ациклические) углеводороды. Алканы Простые вещества
Простые вещества Незвичайна вода
Незвичайна вода Фазовое равновесие
Фазовое равновесие Химия в повседневной жизни человека
Химия в повседневной жизни человека Химическая очистка сточных вод. Окисление и восстановление
Химическая очистка сточных вод. Окисление и восстановление Растворы: состав и их коллигативные свойства
Растворы: состав и их коллигативные свойства Щелочные и щелочноземельные металлы
Щелочные и щелочноземельные металлы Относительная молекулярная масса вещества. Задачи
Относительная молекулярная масса вещества. Задачи Химические свойства карбокатионов
Химические свойства карбокатионов Тіршілік процесіне қатысатын гетерофункционалды қосылыстар
Тіршілік процесіне қатысатын гетерофункционалды қосылыстар Скорость химических реакций
Скорость химических реакций