Содержание
- 2. Atomic Structure Protons, neutrons, electrons How to make ions Relative atomic mass
- 4. 0 -1 +1 1 1 2000 1 9.109 x 10-31 1.602 x 10-19 1.672 x 10-27
- 5. Ionisation Energy What is ionisation energy? Definitions First ionisation energy Successive ionisation energies What affects ionisation
- 6. WHAT IS IONISATION ENERGY? Ionisation Energy is a measure of the amount of energy needed to
- 7. WHAT AFFECTS IONISATION ENERGY? The value of the 1st Ionisation Energy depends on the electronic structure
- 8. Ionisation Energy is affected by 3 things: Atomic Radius Nuclear Attraction Electron Shielding I. E. Decreases
- 9. Successive Ionisation Energies A measure of the energy required to remove each electron in turn. Mg(g)
- 10. Which electron is removed first? (First Ionisation Energy)
- 11. Which electron is removed first? (First Ionisation Energy)
- 12. Successive Ionisation Energies of Calcium Draw a graph to show the successive ionisation energies of calcium,
- 15. 1s2 2s2 2p6 3s2 3p6 4s2 2,8,8,2
- 17. Скачать презентацию
 Нефть и газ
Нефть и газ Студенттің өзіндік жұмысы
Студенттің өзіндік жұмысы Основания, их классификация и свойства в свете теории электролитической диссоциации
Основания, их классификация и свойства в свете теории электролитической диссоциации Правила техники безопасности в химическом кабинете. Правила пользования лабораторным оборудованием и нагревательными приборами
Правила техники безопасности в химическом кабинете. Правила пользования лабораторным оборудованием и нагревательными приборами Кислород. 9 класс
Кислород. 9 класс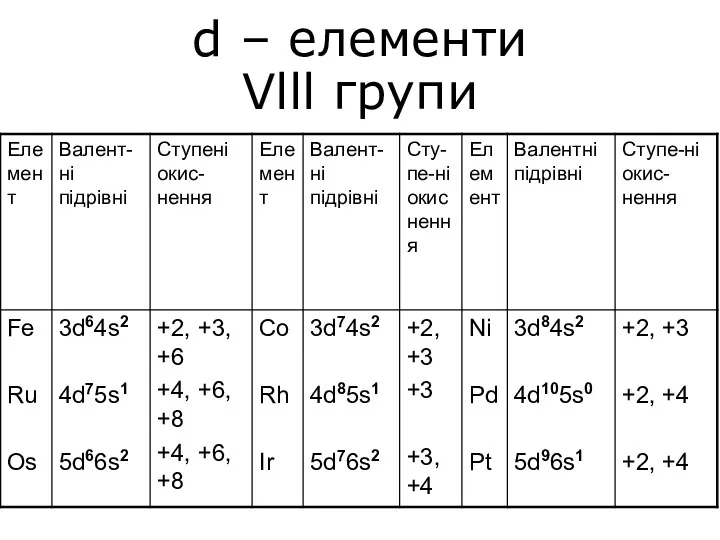 d – елементи Vlll групи
d – елементи Vlll групи Щелочные металлы
Щелочные металлы Поверхностная активность и поверхностно активное вещество
Поверхностная активность и поверхностно активное вещество Химический элемент водород
Химический элемент водород Бытовая химия. Правила безопасного обращения со средствами бытовой химии
Бытовая химия. Правила безопасного обращения со средствами бытовой химии Стирка по научному
Стирка по научному Комплексті қосылыстар және олардың биологиялық маңызы
Комплексті қосылыстар және олардың биологиялық маңызы Деструкция полимеров
Деструкция полимеров Распределение элементов на Земле и в космосе
Распределение элементов на Земле и в космосе Химиядан сұрақтар
Химиядан сұрақтар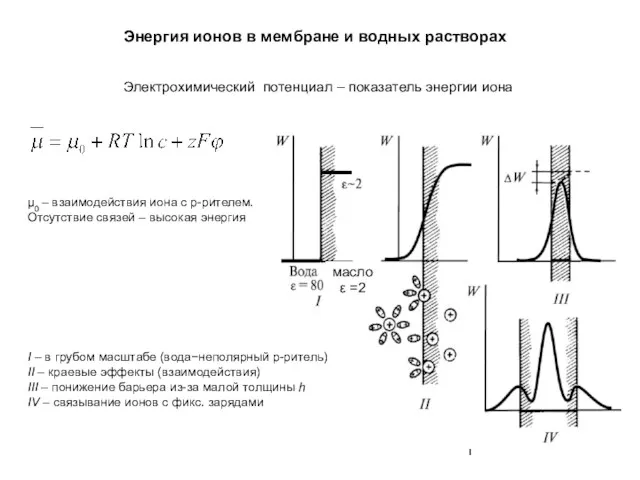 Энергия ионов в мембране и водных растворах
Энергия ионов в мембране и водных растворах Кислотные и основные свойства органических соединений
Кислотные и основные свойства органических соединений Полимеры
Полимеры Изменения, происходящие с белками в процессах технологической переработки сырья
Изменения, происходящие с белками в процессах технологической переработки сырья Цикл трикарбоновых кислот (Ц.Кребса). Подсчёт суммарного энергетического эффекта аэробного окисления глюкозы
Цикл трикарбоновых кислот (Ц.Кребса). Подсчёт суммарного энергетического эффекта аэробного окисления глюкозы Алюминий и его соединения
Алюминий и его соединения Изомерия
Изомерия Химия элементов VIIA группы
Химия элементов VIIA группы Химическая кинетика и катализ механики. (Лекция 5)
Химическая кинетика и катализ механики. (Лекция 5) Физические свойства металлов
Физические свойства металлов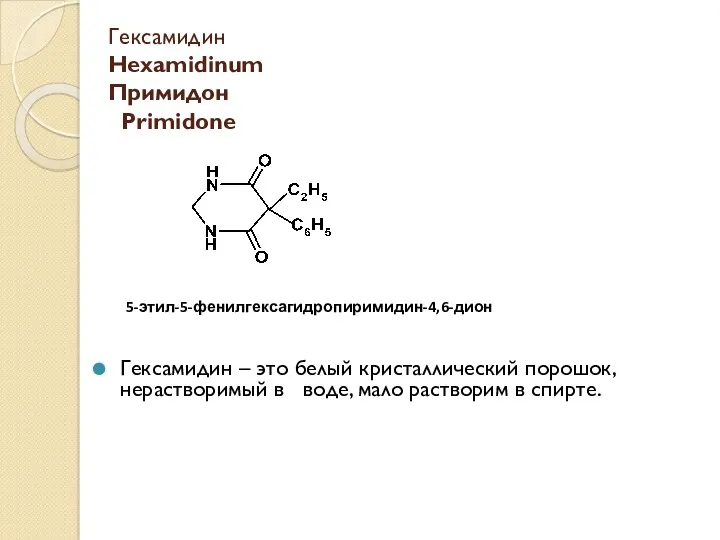 Гексамидин Hexamidinum. Примидон Primidone
Гексамидин Hexamidinum. Примидон Primidone Галогены. Строение атома
Галогены. Строение атома Кислородные соединения серы. 2 часть
Кислородные соединения серы. 2 часть