Содержание
- 2. Learning Objectives Recall the synthesis of chloroalkanes Understand environmental concerns about haloalkanes and understand the mechanism
- 3. Success Criteria Write equations for the synthesis of chloroalkanes and other halogenoalkanes. Gives some examples of
- 4. Keywords Halogenoalkane (haloalkane) Chlorofluorocarbons (CFCs) Primary, secondary, tertiary haloalkanes Free radical substitution Electrophilic addition Initiation, propagation,
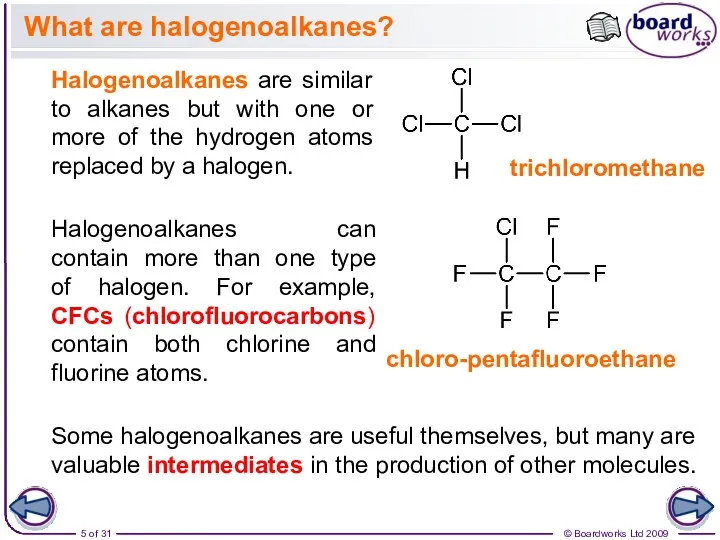
- 5. What are halogenoalkanes? Halogenoalkanes are similar to alkanes but with one or more of the hydrogen
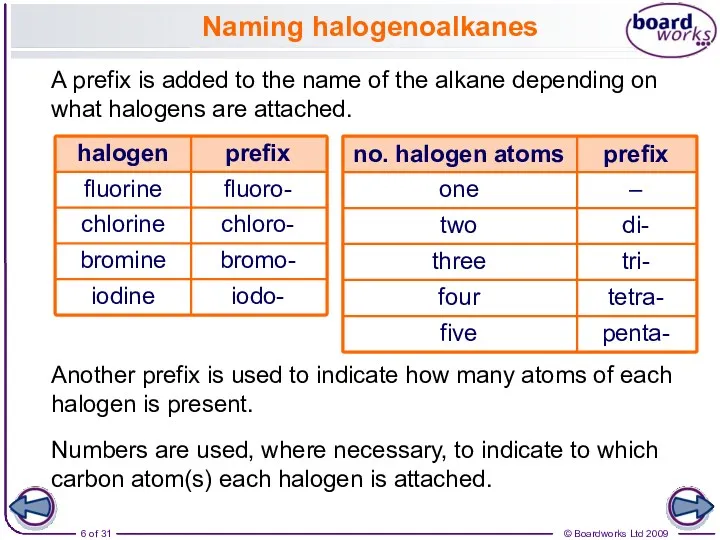
- 6. Naming halogenoalkanes A prefix is added to the name of the alkane depending on what halogens
- 7. What’s the halogenoalkane?
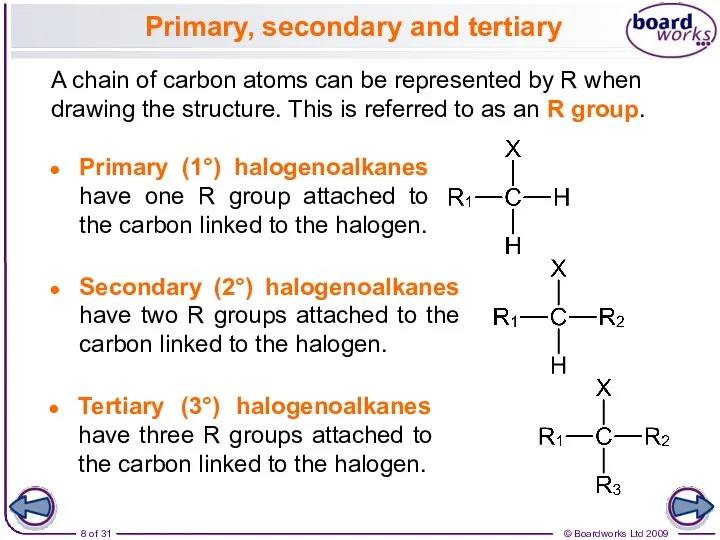
- 8. A chain of carbon atoms can be represented by R when drawing the structure. This is
- 9. Primary, secondary or tertiary?
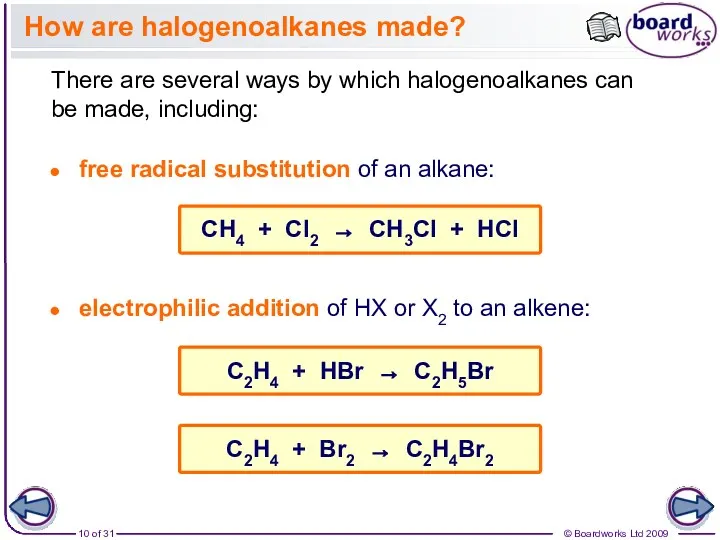
- 10. How are halogenoalkanes made? There are several ways by which halogenoalkanes can be made, including: free
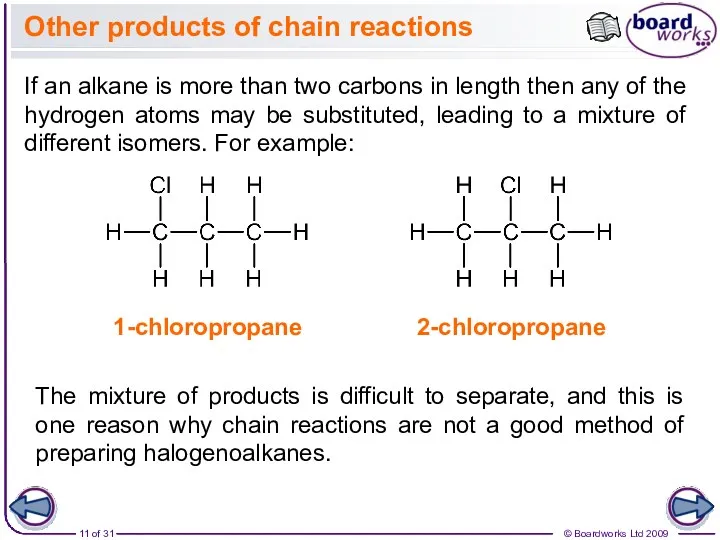
- 11. Other products of chain reactions If an alkane is more than two carbons in length then
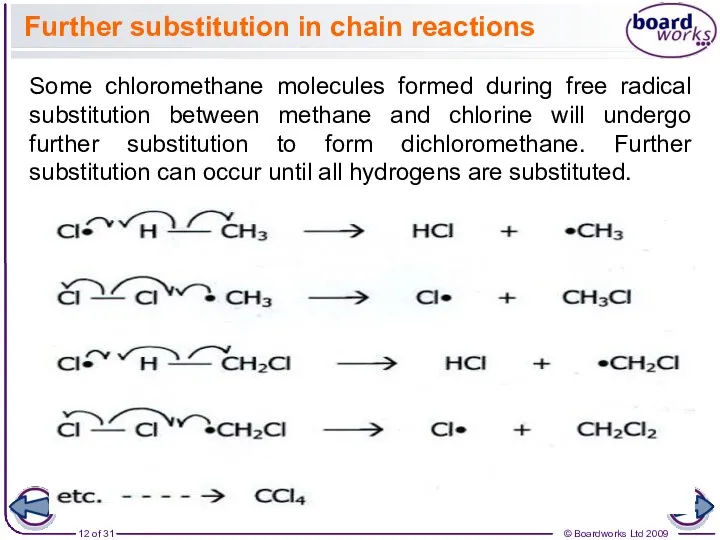
- 12. Further substitution in chain reactions Some chloromethane molecules formed during free radical substitution between methane and
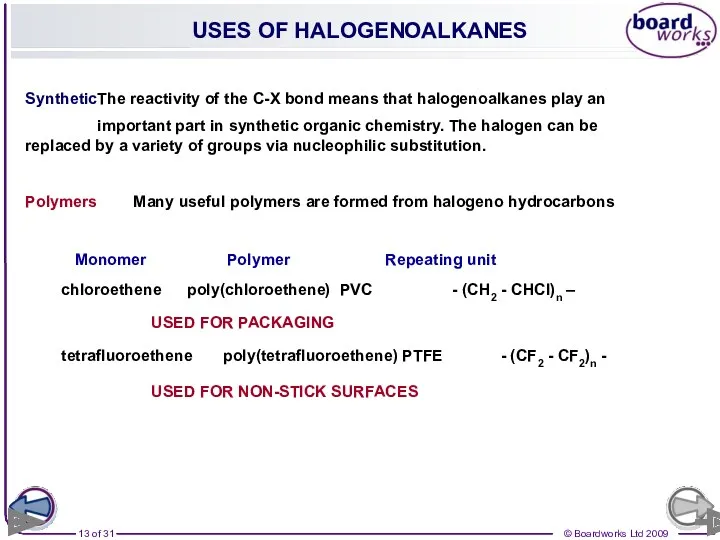
- 13. USES OF HALOGENOALKANES Synthetic The reactivity of the C-X bond means that halogenoalkanes play an important
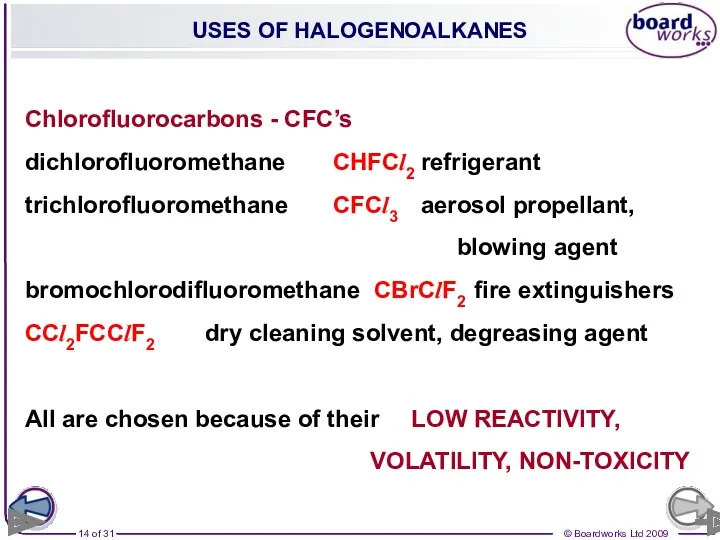
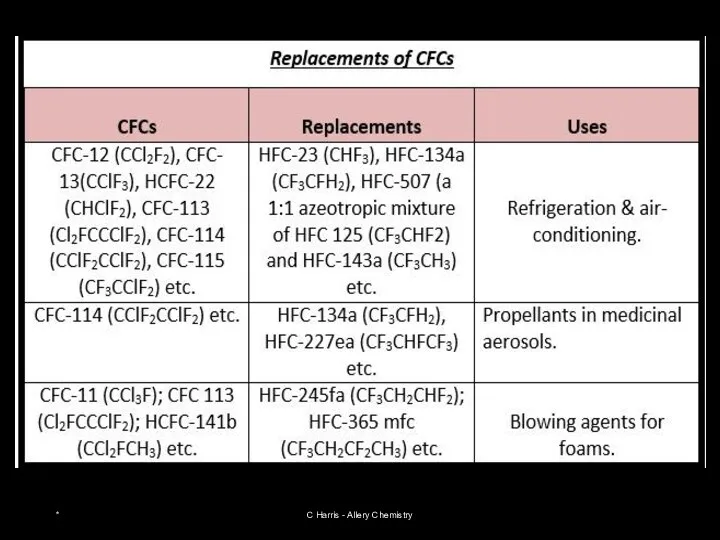
- 14. USES OF HALOGENOALKANES Chlorofluorocarbons - CFC’s dichlorofluoromethane CHFCl2 refrigerant trichlorofluoromethane CFCl3 aerosol propellant, blowing agent bromochlorodifluoromethane
- 15. Benzene hexachloride (BHC) pesticide Dichlorodiphenyltrichloroethane (DDT) Mosquito control Chloroform used to extract and purify penicillin. Was
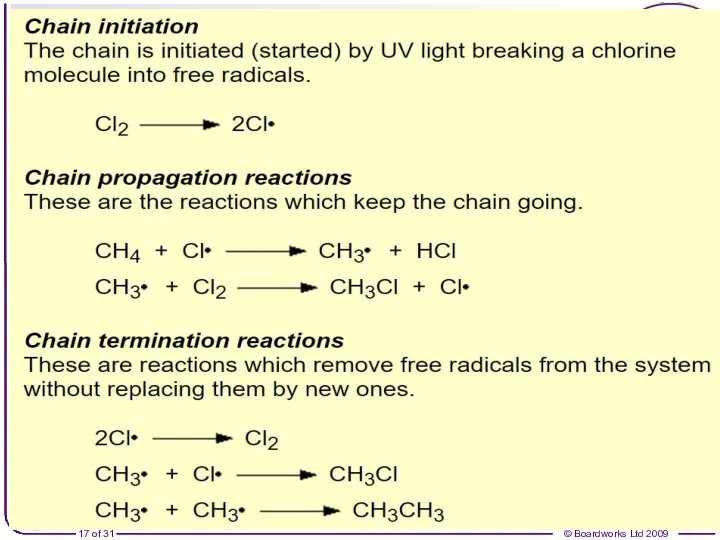
- 16. Free radical substitution: Cl2 + CH4
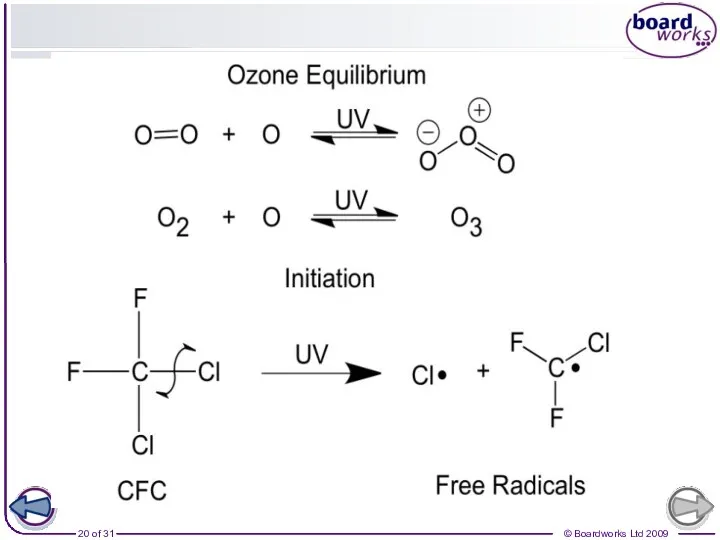
- 19. Chain reactions and ozone
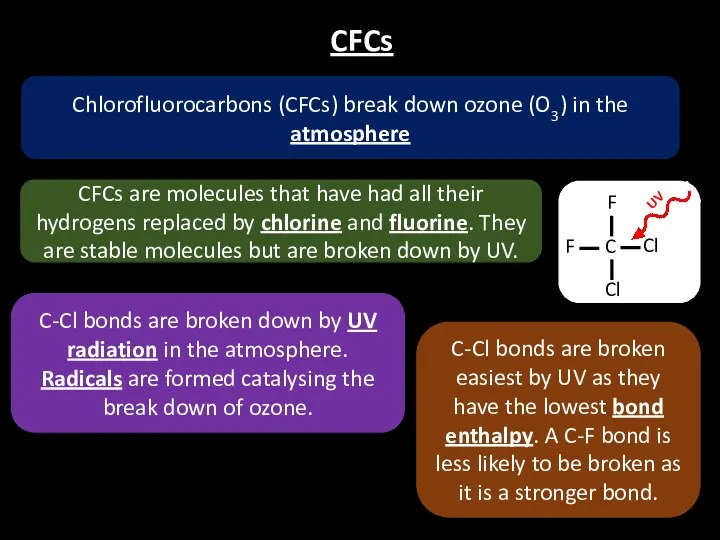
- 21. CFCs CFCs are molecules that have had all their hydrogens replaced by chlorine and fluorine. They
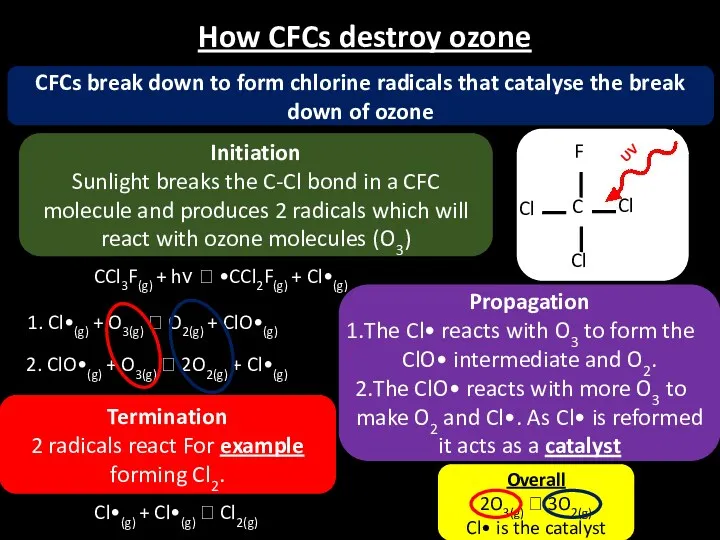
- 22. How CFCs destroy ozone CFCs break down to form chlorine radicals that catalyse the break down
- 23. * C Harris - Allery Chemistry
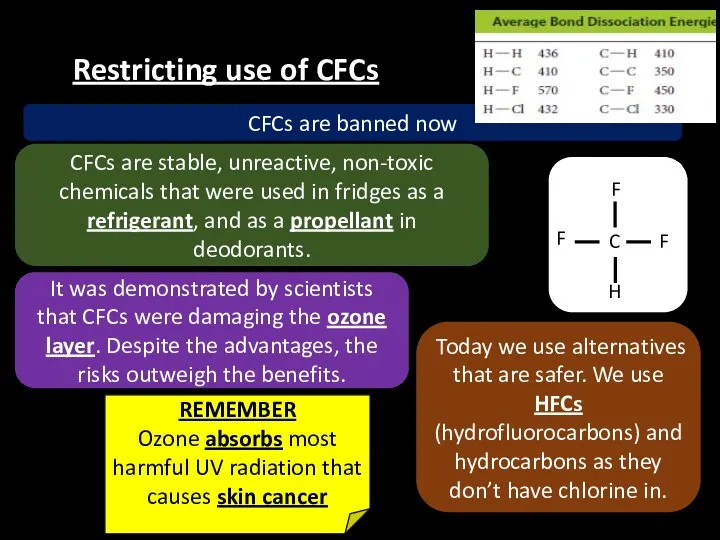
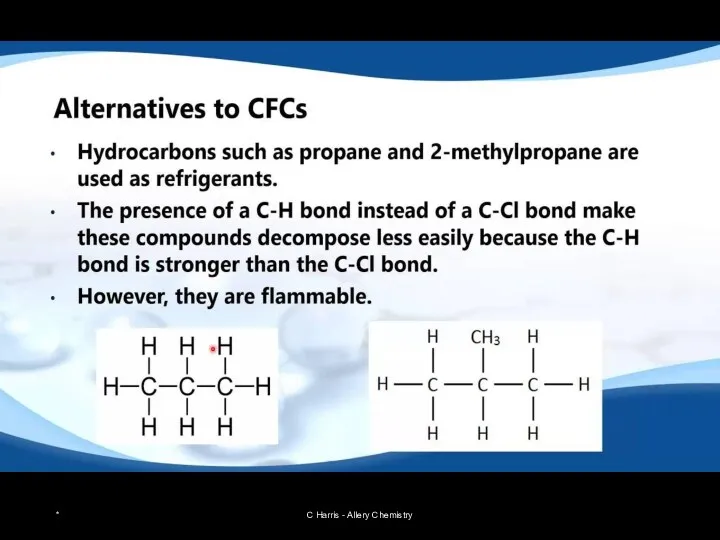
- 24. Restricting use of CFCs CFCs are stable, unreactive, non-toxic chemicals that were used in fridges as
- 25. * C Harris - Allery Chemistry
- 26. * C Harris - Allery Chemistry
- 27. Free radical reactions: true or false?
- 29. Скачать презентацию








 Виявлення в розчині гідроксид-іонів та йонів Гідрогену. Якісні реакції на деякі йони. Застосування якісних реакцій
Виявлення в розчині гідроксид-іонів та йонів Гідрогену. Якісні реакції на деякі йони. Застосування якісних реакцій Хімічні явища в побуті
Хімічні явища в побуті Строение атома
Строение атома Озоновый слой. Механизмы образования и разрушения
Озоновый слой. Механизмы образования и разрушения Point defects. Line defects. Surface Imperfections
Point defects. Line defects. Surface Imperfections Алкины
Алкины Хімічні та фізичні явища
Хімічні та фізичні явища Аналитическая химия. Физико-химические методы анализа
Аналитическая химия. Физико-химические методы анализа Қанықпаған майлар және соның негізіндегі БАЗ
Қанықпаған майлар және соның негізіндегі БАЗ Тотығутотықсыздану титрлеу әдістері. Дәріс № 7
Тотығутотықсыздану титрлеу әдістері. Дәріс № 7 Новое направление в бизнесе компании – катализаторы синтеза метанола
Новое направление в бизнесе компании – катализаторы синтеза метанола Элементы 17 группы
Элементы 17 группы Углерод и его свойства
Углерод и его свойства Презентации-задания к урокам химии по различным темам
Презентации-задания к урокам химии по различным темам Биологически важные пяти- и шестичленные гетероциклы с одним и двумя гетероатомами
Биологически важные пяти- и шестичленные гетероциклы с одним и двумя гетероатомами Особенности сжигания жидкого топлива и топливосжигающие устройства
Особенности сжигания жидкого топлива и топливосжигающие устройства Алюминий
Алюминий Углерод, как химический элемент и простое вещество
Углерод, как химический элемент и простое вещество Жану процесі
Жану процесі Аммиак. Строения молекулы аммиака, его физических и химических свойств
Аммиак. Строения молекулы аммиака, его физических и химических свойств Использование технологии уровневой дифференциации на уроках химии
Использование технологии уровневой дифференциации на уроках химии Химические и физические свойства кремния
Химические и физические свойства кремния Состояние электронов в атоме
Состояние электронов в атоме Технология промышленных газов
Технология промышленных газов Общая геохимия. Изотопы и их использование при решении геологических проблем
Общая геохимия. Изотопы и их использование при решении геологических проблем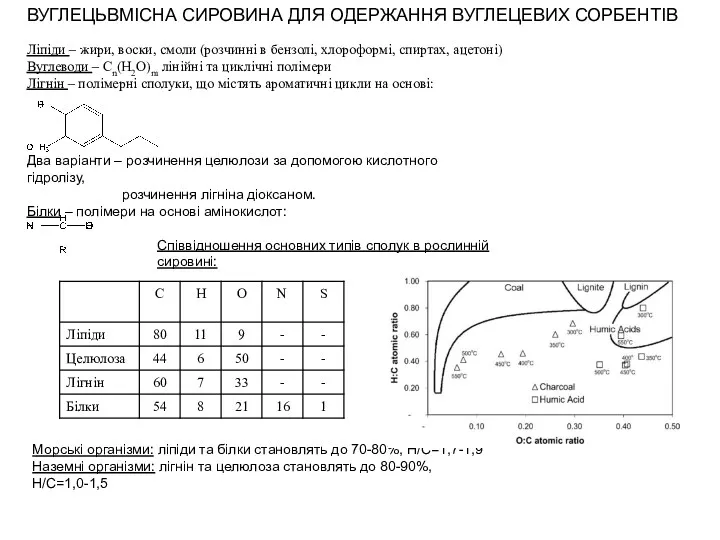 Вуглецьвмісна сировина для одержання вуглецевих сорбентів
Вуглецьвмісна сировина для одержання вуглецевих сорбентів Растворение. Растворы
Растворение. Растворы Функциональные производные углеводородов. Галогенопроизводные углеводородов
Функциональные производные углеводородов. Галогенопроизводные углеводородов