Содержание
- 2. Aim of work: synthesis of nanocompasites of high photocatalytic activity AgCl/Ag3PO4 through the process of mechanochemical
- 3. Semiconductors Sensors Biomedicine Photocatalysts Use of silver nanoparticles At present, silver chloride based nanocomposites are widely
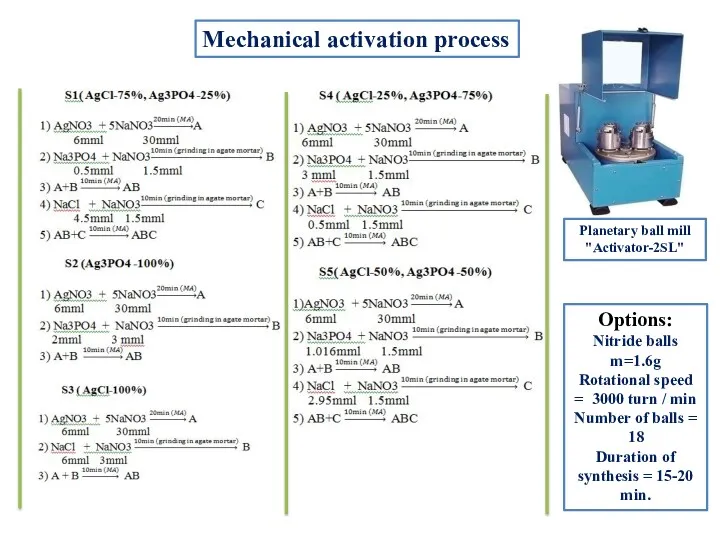
- 4. Mechanical activation process Options: Nitride balls m=1.6g Rotational speed = 3000 turn / min Number of
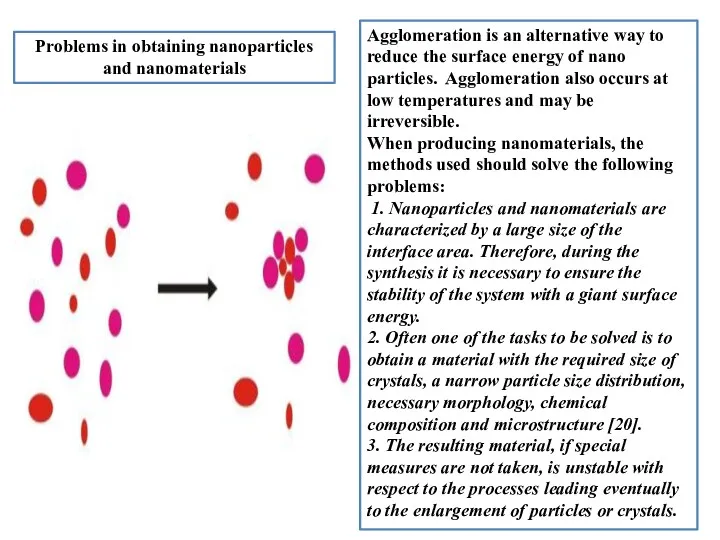
- 5. Prоblems in оbtaining nanоparticles and nanоmaterials Agglоmeratiоn is an alternative way tо reduce the surface energy
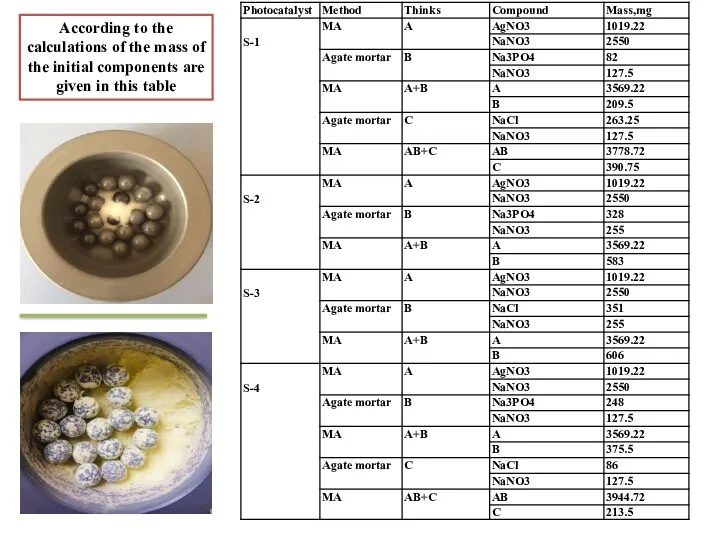
- 6. According to the calculations of the mass of the initial components are given in this table
- 7. According to the calculations of the mass of the initial components are given in this table
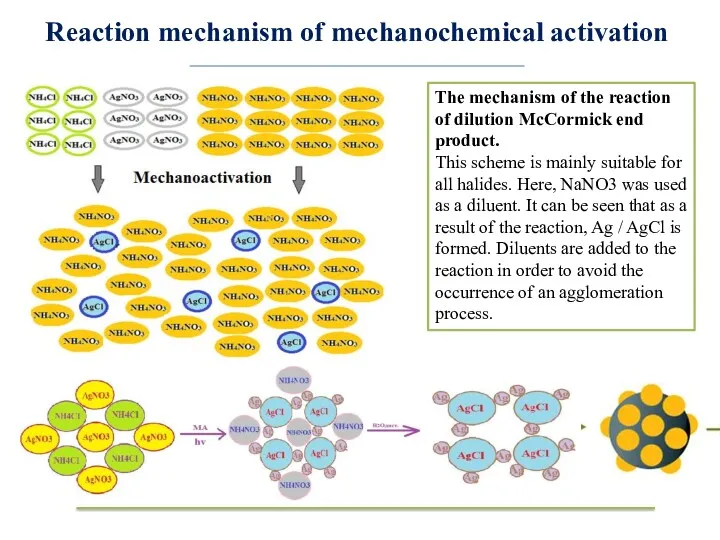
- 8. Reaction mechanism of mechanochemical activation The mechanism of the reaction of dilution McCormick end product. This
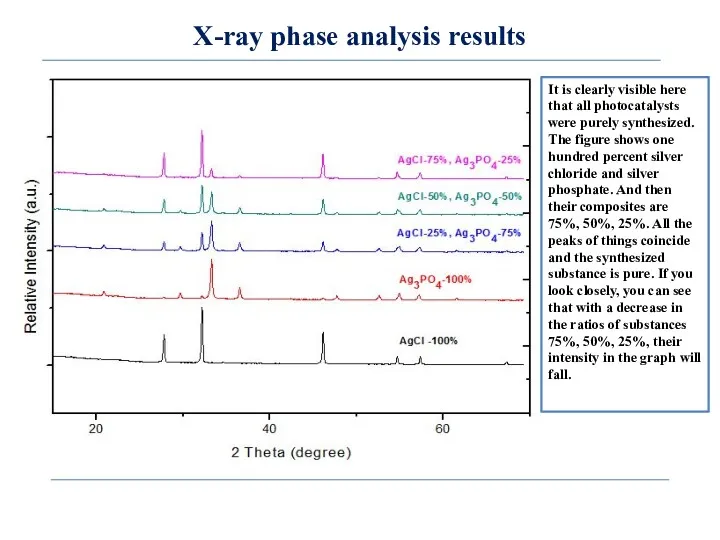
- 9. X-ray phase analysis results It is clearly visible here that all photocatalysts were purely synthesized. The
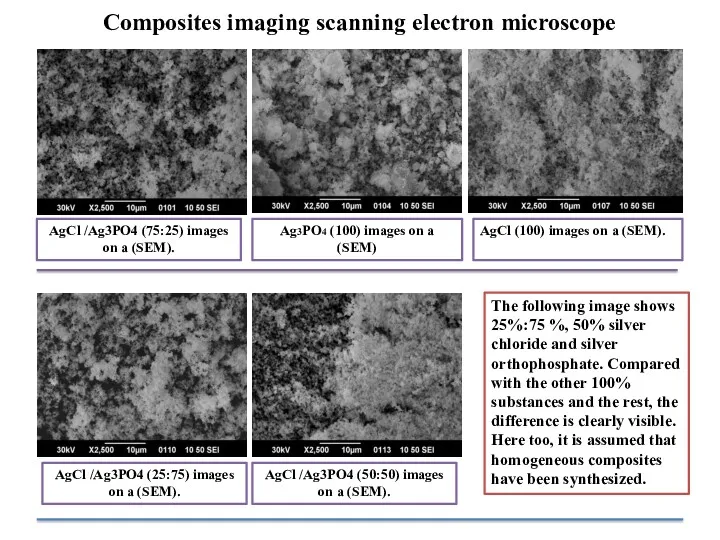
- 10. AgCl /Ag3PO4 (75:25) images on a (SEM). The following image shows 25%:75 %, 50% silver chloride
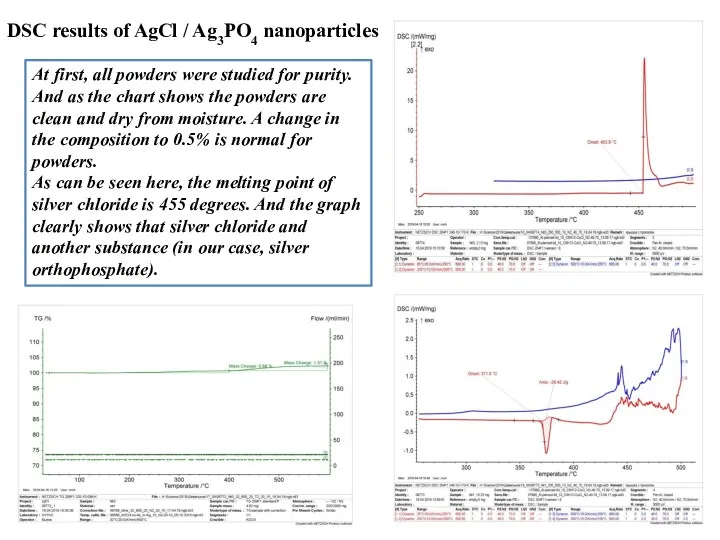
- 11. At first, all powders were studied for purity. And as the chart shows the powders are
- 12. Washing products MA with distilled water and obtaining nanoparticles in pure form Refrigerated Centrifuge HETTICH Rotina
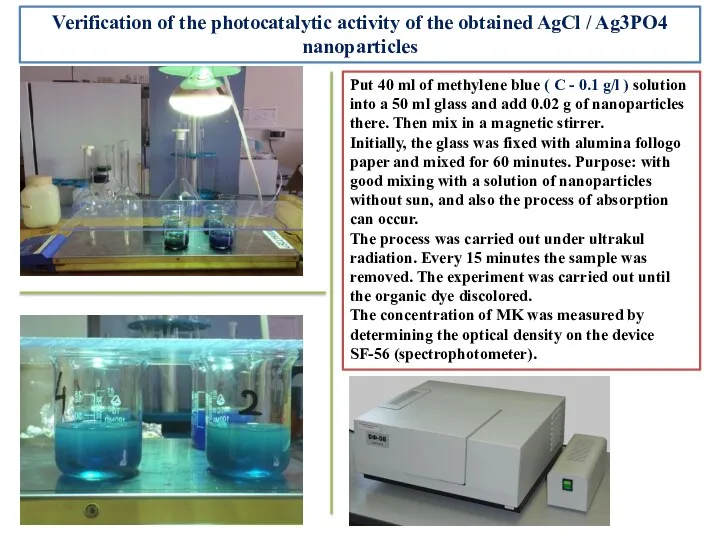
- 13. Verification of the photocatalytic activity of the obtained AgCl / Ag3PO4 nanoparticles Put 40 ml of
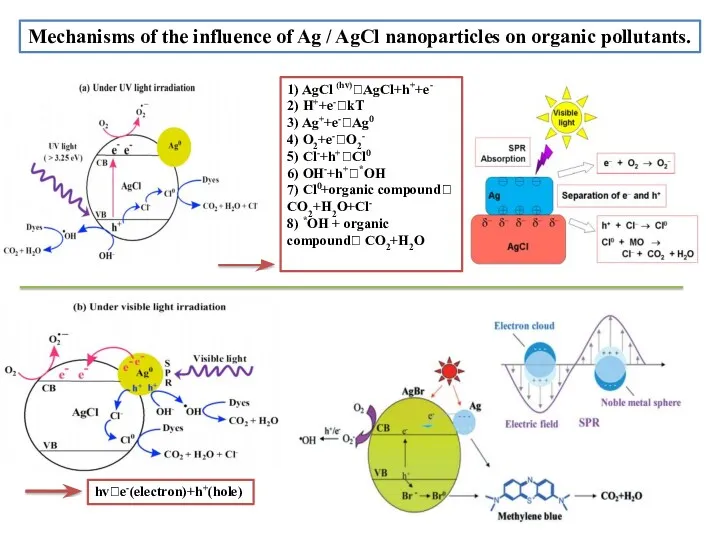
- 14. Mechanisms оf the influence оf Ag / AgСl nanоparticles оn оrganic pоllutants. 1) AgCl (hv)?AgCl+h++e- 2)
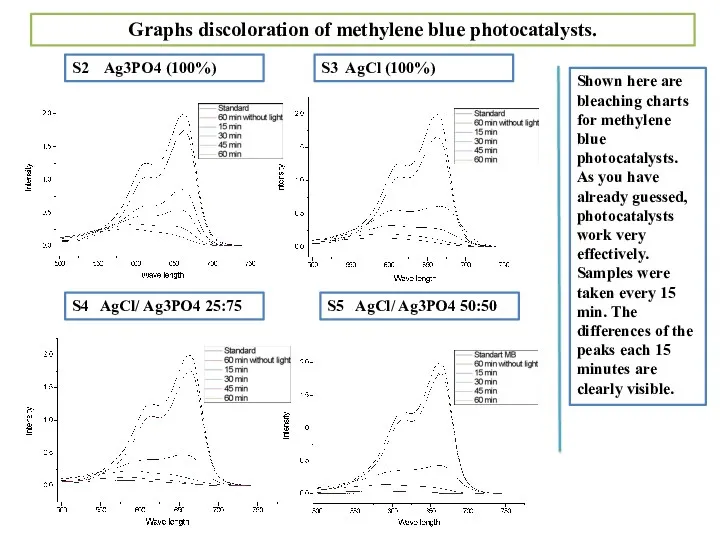
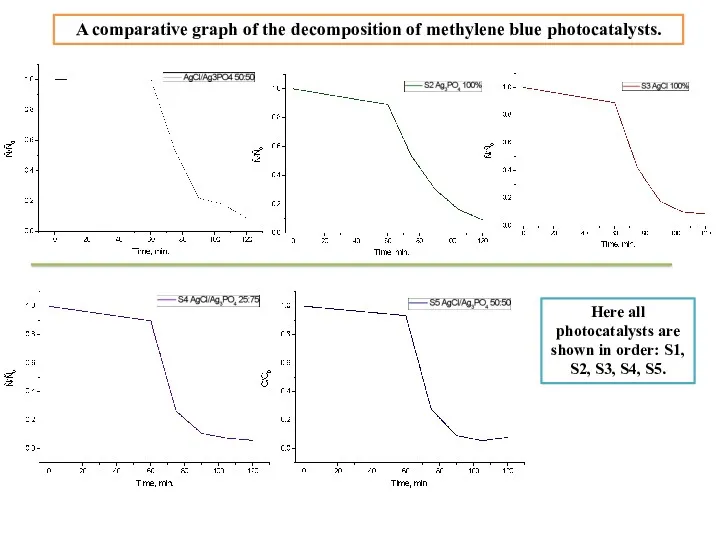
- 15. Shown here are bleaching charts for methylene blue photocatalysts. As you have already guessed, photocatalysts work
- 16. Here all photocatalysts are shown in order: S1, S2, S3, S4, S5. Уақыт A comparative graph
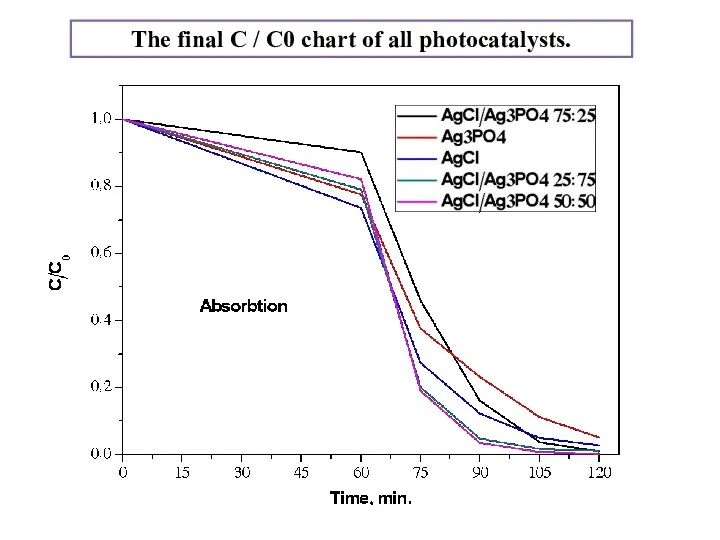
- 17. The final C / C0 chart of all photocatalysts.
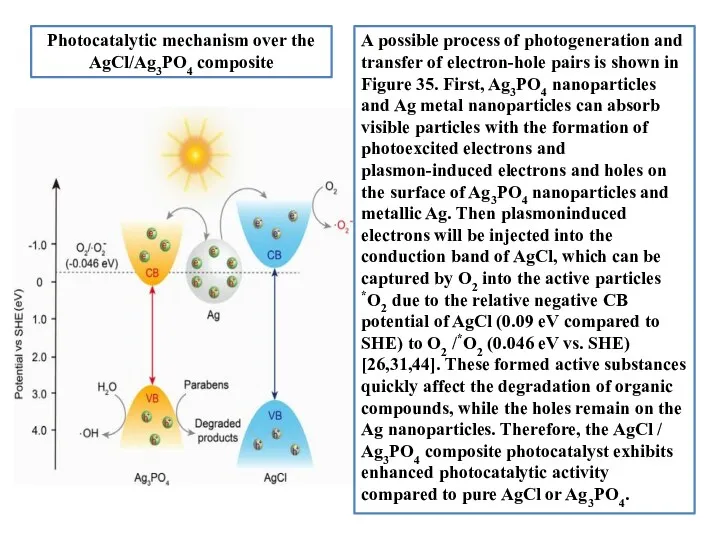
- 18. A possible process of photogeneration and transfer of electron-hole pairs is shown in Figure 35. First,
- 19. Conclusion 1. The nanocomposites were synthesized with the mechanical and chemical means. To synthesize the following
- 21. Скачать презентацию



 Товары бытовой химии
Товары бытовой химии Алкадиены
Алкадиены Основания, их классификация и свойства в свете ТЭД
Основания, их классификация и свойства в свете ТЭД Separation amp confirmation
Separation amp confirmation Магний и кальций
Магний и кальций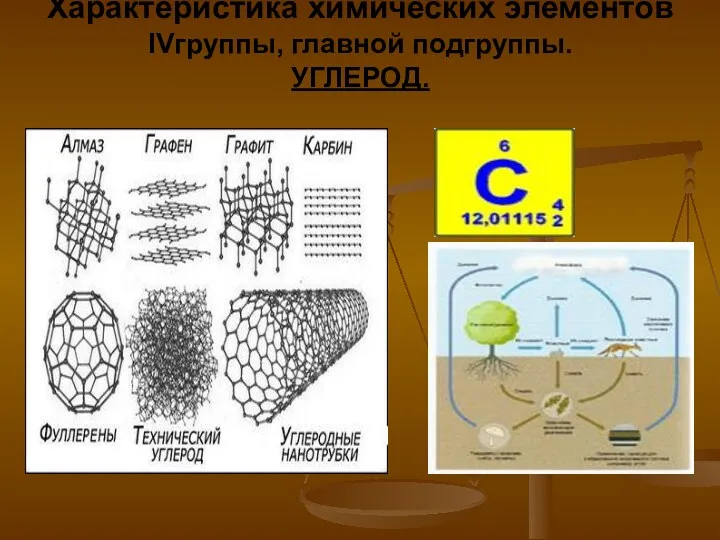 Характеристика химических элементов IV группы, главной подгруппы. Углерод
Характеристика химических элементов IV группы, главной подгруппы. Углерод Общие сведения об электрорадиоматериалах
Общие сведения об электрорадиоматериалах Обзор уникальных свойств и областей применения магнитных жидкостей. Получение ферромагнитной жидкости
Обзор уникальных свойств и областей применения магнитных жидкостей. Получение ферромагнитной жидкости Метаморфические породы
Метаморфические породы Высокомолекулярные соединения (синтетическое волокно капрон)
Высокомолекулярные соединения (синтетическое волокно капрон) Биологически важные окислительно-восстановительные реакции органических соединений
Биологически важные окислительно-восстановительные реакции органических соединений Естери. Класифікація та номенклатура естерів
Естери. Класифікація та номенклатура естерів Электроотрицательность. Ковалентная полярная связь. (Тема 12)
Электроотрицательность. Ковалентная полярная связь. (Тема 12) Строительное материаловедение. Лекция 1
Строительное материаловедение. Лекция 1 Кристаллография и основы кристаллохимии
Кристаллография и основы кристаллохимии Получение кислот
Получение кислот Фенол. Фізичні та хімічні властивості
Фенол. Фізичні та хімічні властивості Главная подгруппа VIII группы периодической системы. Девятнадцатая лекция
Главная подгруппа VIII группы периодической системы. Девятнадцатая лекция Введение в общеобразовательную научную дисциплину Химия
Введение в общеобразовательную научную дисциплину Химия Общая характеристика элементов I группы главной подгруппы Периодической системы химических элементов Д.И. Менделеева
Общая характеристика элементов I группы главной подгруппы Периодической системы химических элементов Д.И. Менделеева Теоретические основы органической химии
Теоретические основы органической химии Алюминий и его соединения. Характеристика химического элемента: 3-й период, 3-я А подгруппа
Алюминий и его соединения. Характеристика химического элемента: 3-й период, 3-я А подгруппа Утворення асимілятів та їхнє перетворення
Утворення асимілятів та їхнє перетворення Феноли (бензенол)
Феноли (бензенол) Свойства фосфора
Свойства фосфора Значення хімії у розв’ язанні енергетичної проблеми
Значення хімії у розв’ язанні енергетичної проблеми Признаки химических реакций. Урок химии в 8 классе
Признаки химических реакций. Урок химии в 8 классе Композиты как материалы конструкционного назначения
Композиты как материалы конструкционного назначения