Содержание
- 2. Aim Learn to prepare gaseous samples with desired concentration of a solute
- 3. Importance Preparation of calibration samples (standards) Conducting chemical reactions in gas phase Production of commercial gases
- 4. Advantages of having the skill More accurate calibration and analytical measurements Lower consumption of expensive materials
- 5. Example - quantification
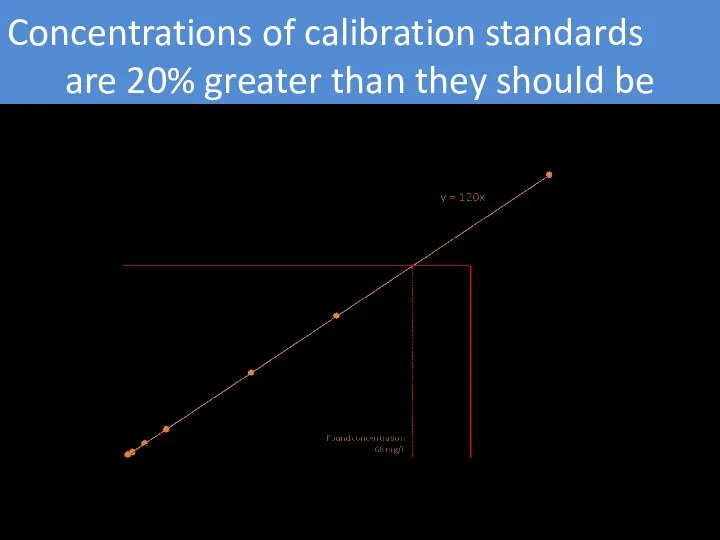
- 6. Concentrations of calibration standards are 20% greater than they should be
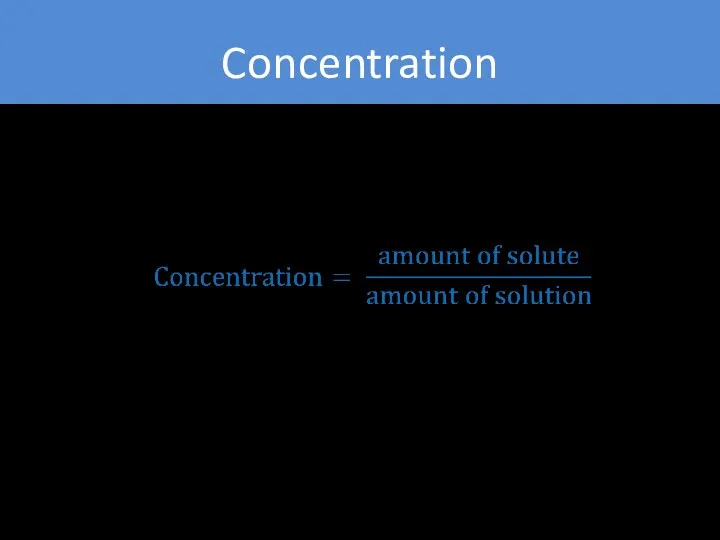
- 7. Concentration general measurement unit stating the amount of solute present in a known amount of solution
- 8. Units of concentrations of gases Liquid samples: volume %; mol/L; g/L; ppm (w/v); ppb (w/v); ppt
- 9. Types of concentrations Volume/volume – does not change with T and P Mass / volume –
- 10. Main formula for conversions p – pressure (ambient or partial), kPa V – volume, L m
- 11. Exercise Convert 50 ppm (v/v) of hydrogen sulfide in air to mg/m3 Now we need to
- 12. Solution (continued) V = 50 mL; R=8.31 L· kPa / (moL K); M (H2S) = 34
- 13. Solution (continued) Q: will the C increase if temperature is increased to 30 ⁰C?
- 14. Question What is the partial pressure of H2S at this concentr.? m = 0.0655 g; V
- 15. Quiz 1/2 1 – 37 2 – 55 3 – 25 4 - 43 5 –
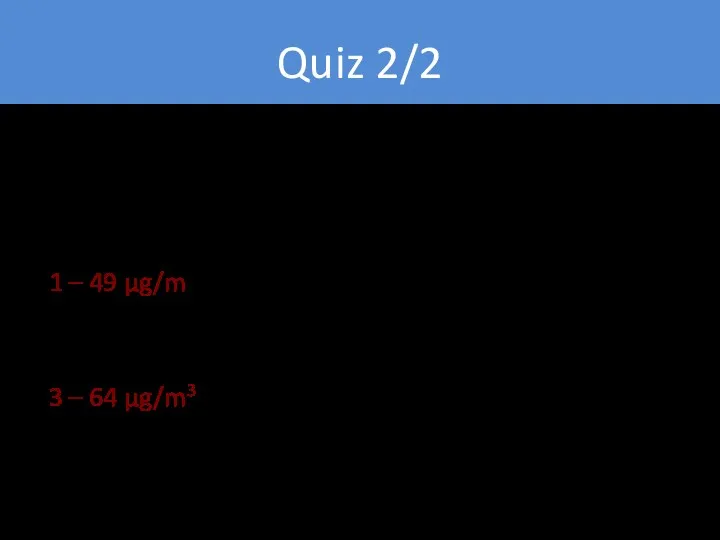
- 16. Quiz 2/2 1 – 49 µg/m3 2 – 56 µg/m3 3 – 64 µg/m3 4 -
- 17. Question What equipment and glassware is used for preparing liquid solutions?
- 18. Calibrated gas sampling bulb To prepare gas standard, inject small amount (
- 19. Exercise How many nanograms of naphthalene should be injected into a 500-mL bulb filled with “zero”
- 20. Exercise (continued) What concentration should the injected solution have if the injected volume is 5.0 µL?
- 21. Exercise Solution of benzene (5.00 µL) in methanol with concentration 10 mg/mL was injected to calibrated
- 22. Task Convert this concentration to ppmV Convert this concentration to Pa
- 23. Question How many microliters of water can be introduced to a 250-mL flask containing dry air
- 24. Task 2 How many microliters of methanol can be introduced to a 250-mL flask containing air
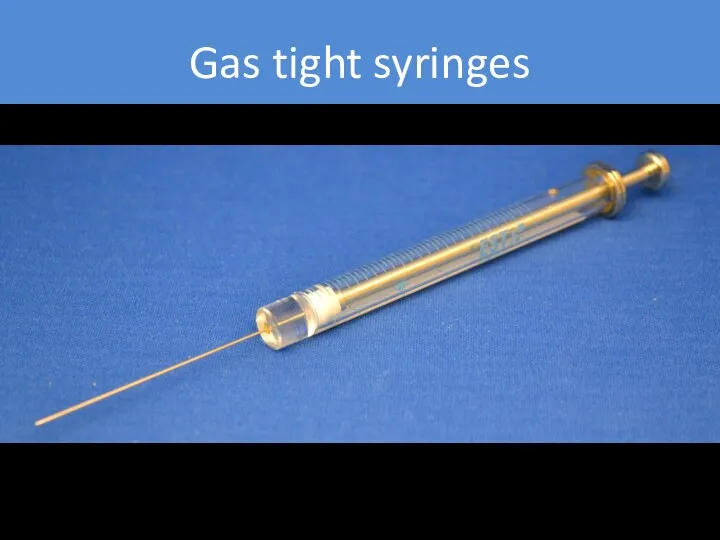
- 25. Gas tight syringes PTFE plunger
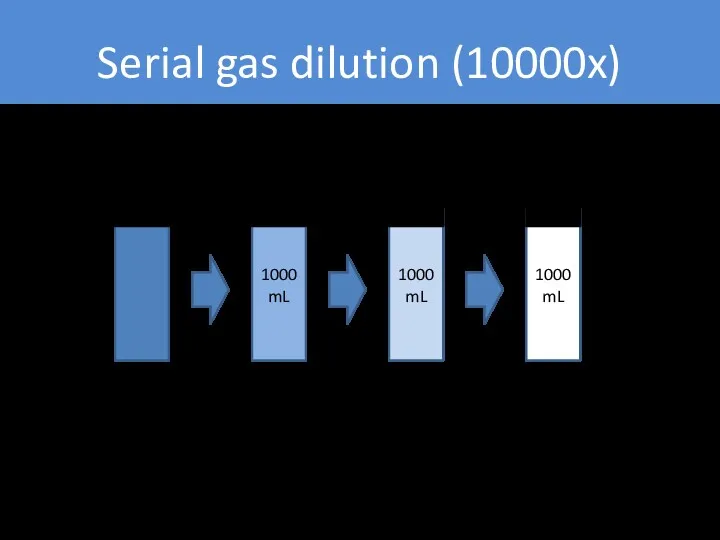
- 26. Serial gas dilution (10000x) 1000 mL 1000 mL 1000 mL Pure gas 100 µL/L (100 ppm)
- 27. Method 2 C = 0 C > 0 Tuduri et al., 2001
- 28. New Era NE-1002X
- 29. Example “Zero” air is supplied at 100 mL/min rate Benzene solution in methanol (C = 50
- 30. Calculation
- 32. Скачать презентацию












 Методи добування у промисловості
Методи добування у промисловості Электротехнический фарфор – разновидность твердого фарфора
Электротехнический фарфор – разновидность твердого фарфора Растворы. Смеси веществ
Растворы. Смеси веществ Катализ органических реакций. (Лекция 15)
Катализ органических реакций. (Лекция 15)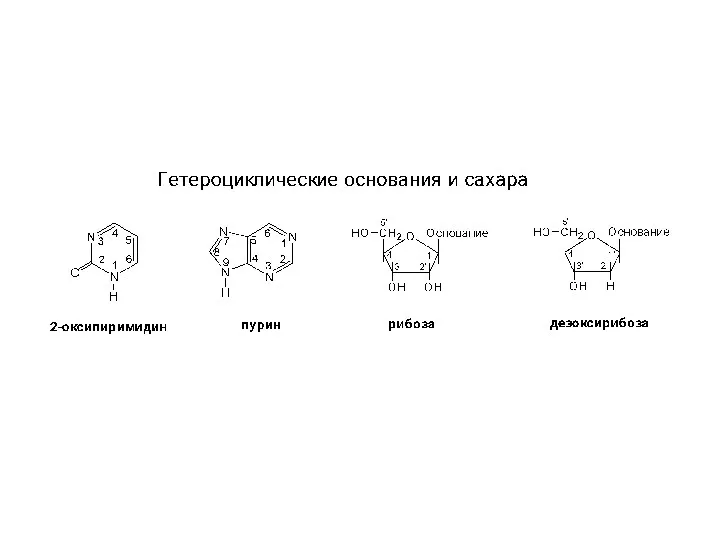 Структура гетероциклов, нуклеозидов и нуклеотидов
Структура гетероциклов, нуклеозидов и нуклеотидов Азотные удобрения
Азотные удобрения Гомологический ряд алканов. Изомерия и номенклатура
Гомологический ряд алканов. Изомерия и номенклатура Биологически важные гетероциклы
Биологически важные гетероциклы Записать формулы
Записать формулы Интересные факты о химических веществах
Интересные факты о химических веществах Нуклеопротеины
Нуклеопротеины Характеристика s,p,d,f - элементов
Характеристика s,p,d,f - элементов Физико-химия дисперсных систем. Коллоидные растворы
Физико-химия дисперсных систем. Коллоидные растворы Сполуки неметалічних елементів з Гідрогеном
Сполуки неметалічних елементів з Гідрогеном Ароматические соединения. Лекция 10
Ароматические соединения. Лекция 10 Кислородные соединения азота
Кислородные соединения азота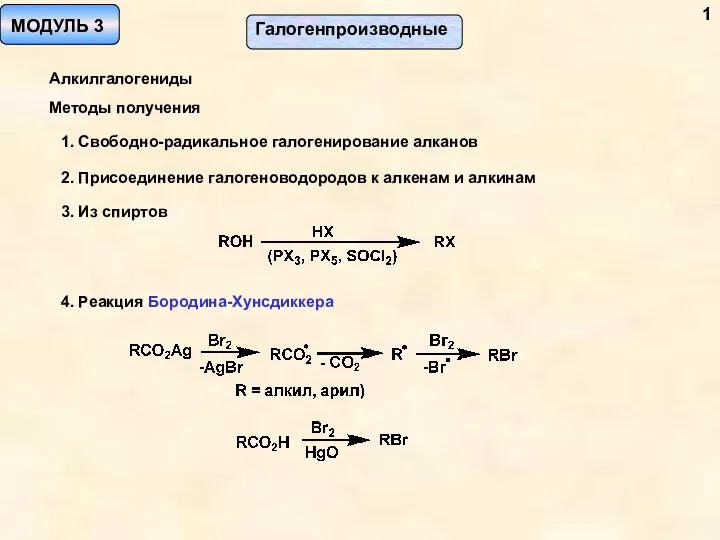 Галогенпроизводные. Алкилгалогениды. Методы получения. (Модуль 3)
Галогенпроизводные. Алкилгалогениды. Методы получения. (Модуль 3) Органические соединения серы
Органические соединения серы Спирттер мен фенолдар
Спирттер мен фенолдар Экспериментальные методы физико-химических исследований. Лекция 7
Экспериментальные методы физико-химических исследований. Лекция 7 Способы выражения состава раствора. Задача 7
Способы выражения состава раствора. Задача 7 Двойной электрический слой. Теория Гельмгольца
Двойной электрический слой. Теория Гельмгольца Ароматические углеводороды (арены)
Ароматические углеводороды (арены) Сущность процесса электролитической диссоциации
Сущность процесса электролитической диссоциации Химическая и электрическая работа систем с химическими реакциями. Устройства для проведения электрохимических реакций
Химическая и электрическая работа систем с химическими реакциями. Устройства для проведения электрохимических реакций Алюминий и его соединения
Алюминий и его соединения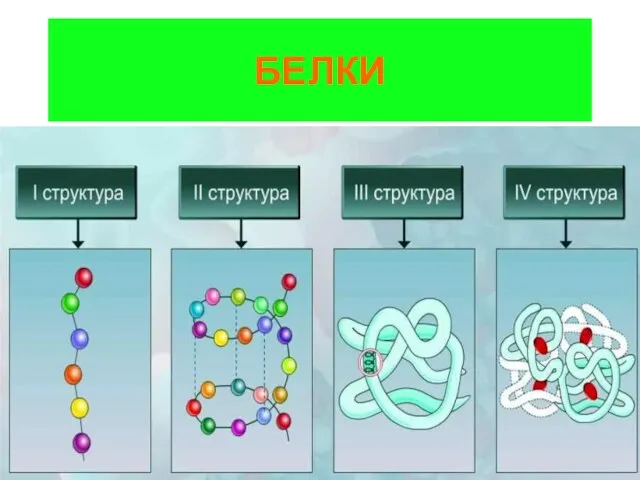 Белки. Строение
Белки. Строение Chemical potential. Chemical potential of an ideal gas
Chemical potential. Chemical potential of an ideal gas