Содержание
- 2. LEARNING OBJECTIVES 11.2.2.7 understand the purpose of, be able to carry out, and be able to
- 3. The Titration One of the most important lab procedures involving acids and bases is the titration.
- 4. Acid-Base Titration Terms to Know Titrant: the standard solution of known molarity in the buret that
- 5. Types of Acid-Base Titrations The quality of the titration depends on the strength of the acids
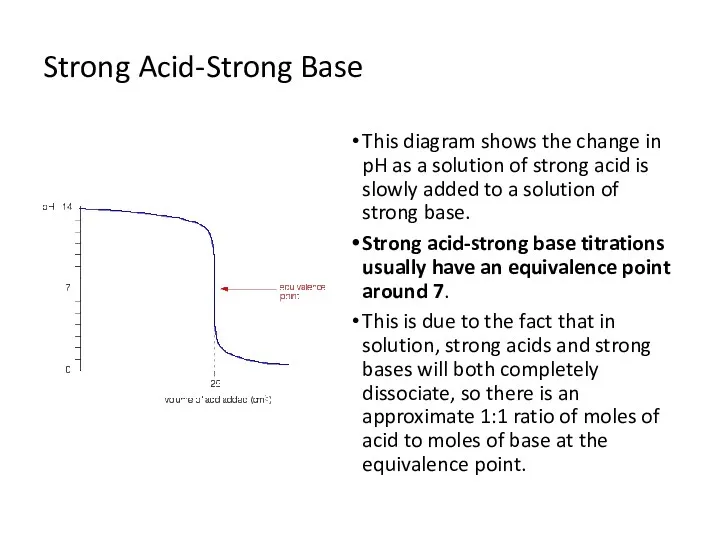
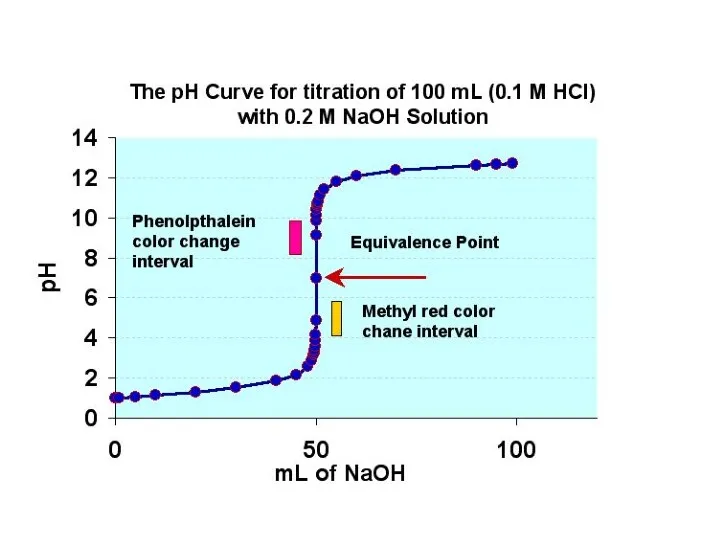
- 6. Strong Acid-Strong Base This diagram shows the change in pH as a solution of strong acid
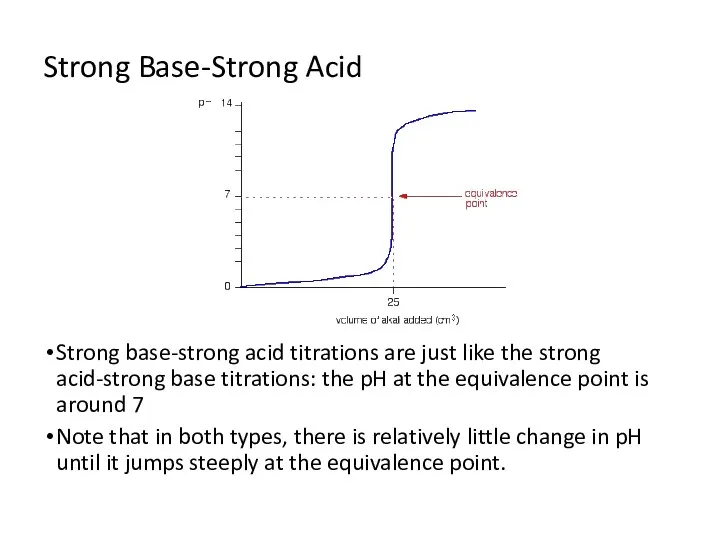
- 7. Strong Base-Strong Acid Strong base-strong acid titrations are just like the strong acid-strong base titrations: the
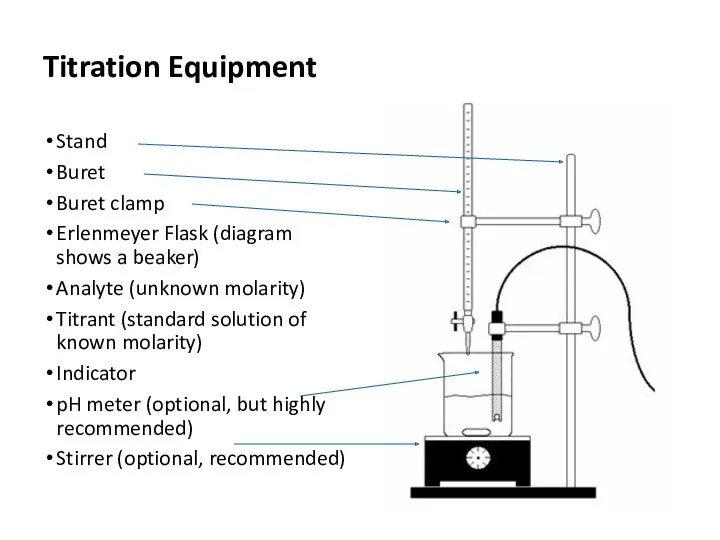
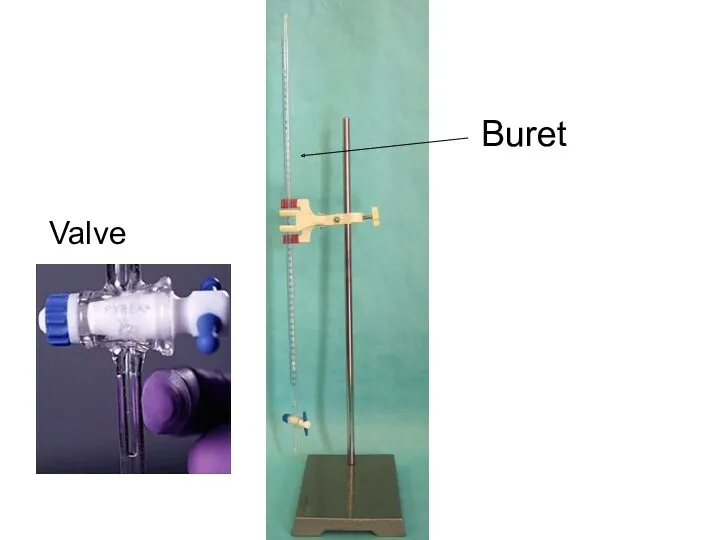
- 8. Titration Equipment Stand Buret Buret clamp Erlenmeyer Flask (diagram shows a beaker) Analyte (unknown molarity) Titrant
- 9. Preparing the Titration Make sure your equipment is clean! Take care when preparing the buret. Run
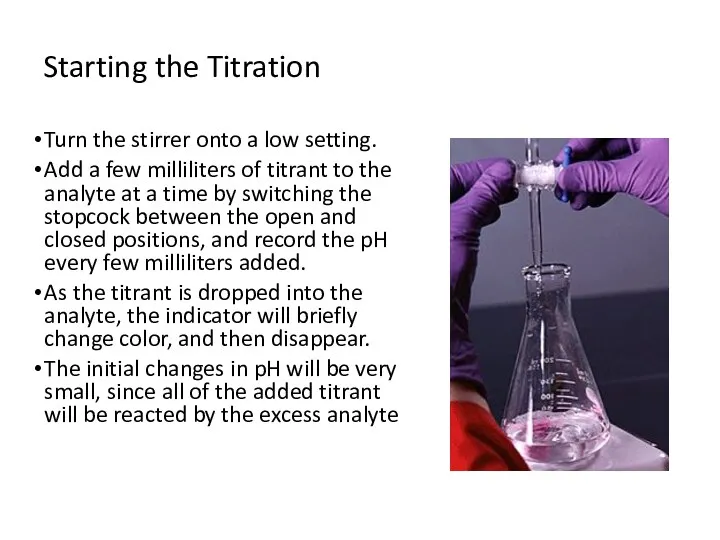
- 10. Starting the Titration Turn the stirrer onto a low setting. Add a few milliliters of titrant
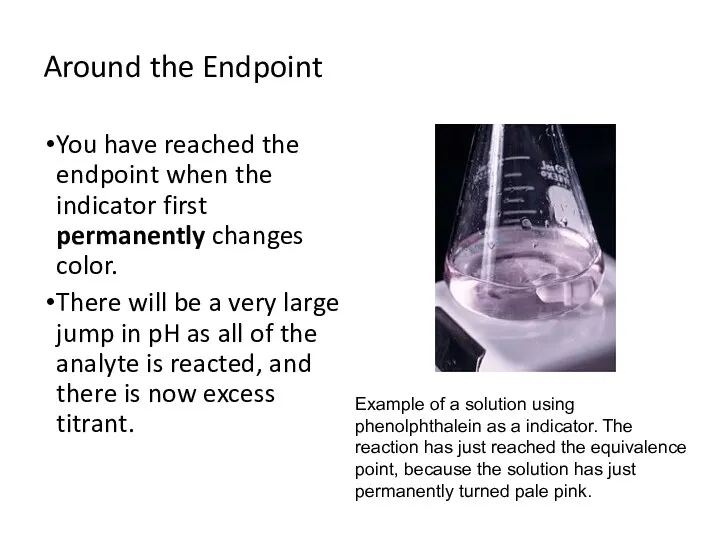
- 11. Around the Endpoint You have reached the endpoint when the indicator first permanently changes color. There
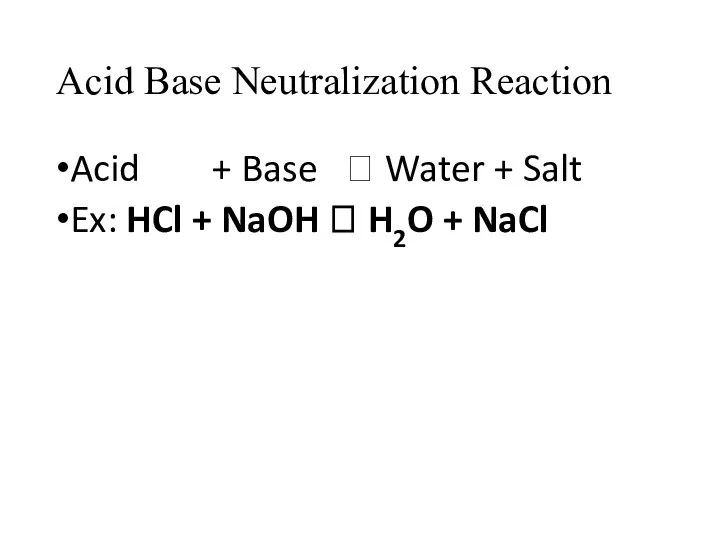
- 12. Acid Base Neutralization Reaction Acid + Base ? Water + Salt Ex: HCl + NaOH ?
- 13. Example: Stomach antacids
- 14. Titration: A laboratory method for determining the concentration of an unknown acid or base using a
- 15. Equivalence Point The point at which there are stoichiometrically equivalent amounts of acid and base. [H+]
- 16. Buret Valve
- 17. Titration Acid with Phenolpthalein End-Point
- 19. Indicators Indicators are chosen, such that they change colors at the range of the pH of
- 20. Methods of Solving Titration Problems: a) using stoichiometry b) using the titration formula aMaVa=bMbVb.
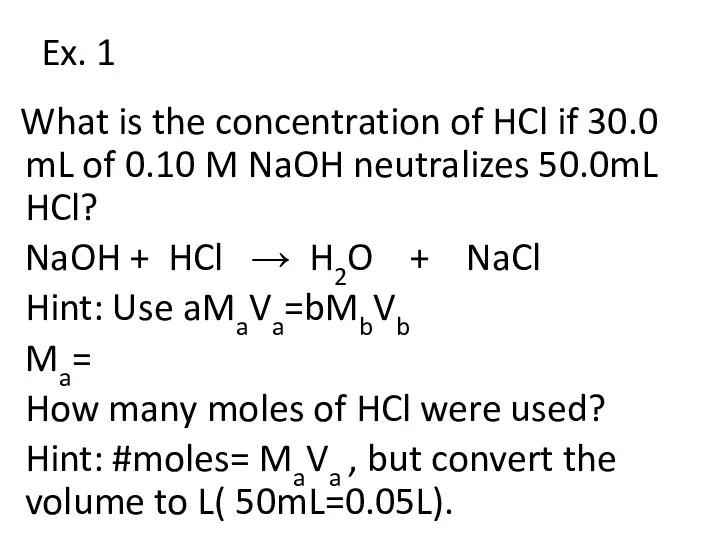
- 21. Ex. 1 What is the concentration of HCl if 30.0 mL of 0.10 M NaOH neutralizes
- 22. Ex. 2 A 20.0 mL solution of Sr(OH)2 is neutralized after 25.0 mL of standard 0.05
- 23. Ex. 3 How many mL of 0.20 M H3PO4 are needed to neutralize 55.0 mL of
- 25. Скачать презентацию












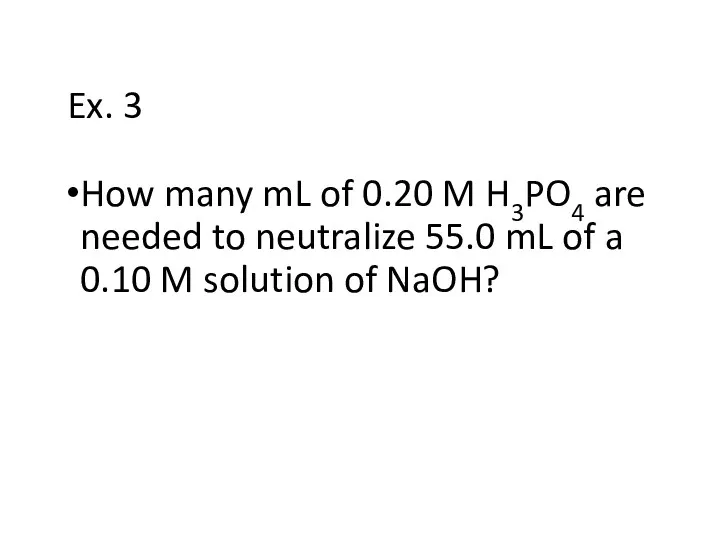
 Хімічні властивості кислот
Хімічні властивості кислот Радикальные реакции. (Лекция 9)
Радикальные реакции. (Лекция 9) Полимерлер-біздің болашағымыз
Полимерлер-біздің болашағымыз Почему нефть называют черным золотом
Почему нефть называют черным золотом Карбоновые кислоты. Изомерия. Физические, химические свойства. Получение, применение
Карбоновые кислоты. Изомерия. Физические, химические свойства. Получение, применение Производство водорода
Производство водорода Общие свойства металлов
Общие свойства металлов Основные положения теории растворов электролитов, используемых в аналитической химии. (Лекция 3)
Основные положения теории растворов электролитов, используемых в аналитической химии. (Лекция 3) Растворы электролитов и неэлектролитов. Ионное произведение воды
Растворы электролитов и неэлектролитов. Ионное произведение воды Минералы и горные породы
Минералы и горные породы Enantioselective Total Synthesis
Enantioselective Total Synthesis Основы химической термодинамики и кинетики химических реакций
Основы химической термодинамики и кинетики химических реакций Общая характеристика галогенов
Общая характеристика галогенов Алканы. Получение, свойства и применение
Алканы. Получение, свойства и применение Термодинамика химических процессов
Термодинамика химических процессов Углеводы (особенности строения, реакционной способности и методы синтеза альдегидо- и кетоспиртов)
Углеводы (особенности строения, реакционной способности и методы синтеза альдегидо- и кетоспиртов) Свойства воды. Оценка качества
Свойства воды. Оценка качества Вычисление массы растворённого вещества, содержащегося в определённой массе раствора с известной массовой долей
Вычисление массы растворённого вещества, содержащегося в определённой массе раствора с известной массовой долей Гетерогенді химиялық реакциялар
Гетерогенді химиялық реакциялар Кобальт. Нахождение в природе. Получение
Кобальт. Нахождение в природе. Получение Группа галогенов в периодической системе
Группа галогенов в периодической системе Подгруппа азота
Подгруппа азота Липиды. Классификация
Липиды. Классификация Химический элемент титан
Химический элемент титан Металлы в природе. Получение
Металлы в природе. Получение Кислоты. Состав кислот
Кислоты. Состав кислот Курс биохимии. Биохимия крови
Курс биохимии. Биохимия крови Гетерофункциональные соединения
Гетерофункциональные соединения