Слайд 2Chemical composition of living things
98% H,O,C,N (bioelements)
~2% S, P, Na, Cl, Ca, K,
Mg, Fe (macroelements)
~0.02% I, F, Co, Mn, Mo, B, Zn (microelements)
In very small amounts Ag, Se, Hg(ultramicroelements)
Слайд 3Chemical reactions
A compound is formed when molecules are rearranged or bonds form between
atoms. The formation of bonds is termed a chemical reaction
Слайд 4Types of reaction
Oxidation - reduction (redox) reactions
Anabolic - catabolic reactions
Hydrolysis -
dehydration synthesis
Слайд 5Oxidation - reduction (redox) reactions
A chemical reaction involves physical changes to all the
reactants involved. For example, a compound may receive or donate electrons. Such reactions are known as oxidation-reduction reactions or redox reactions. The compound donating electrons is said to be oxidised while the compound accepting electrons is said to be reduced.
Ex:
C6H12O6 + 6O2 ⎯→ 6CO2 + 6H2O + Energy (Oxidation of glucose)
Слайд 6Anabolic - Catabolic Reactions
Catabolic Reactions
Organic compounds are broken down to their monomers
by catabolic reactions, most of which result in energy release.
EX: C6H12O6 + 6O2 ⎯→ 6CO2 + 6H2O + Energy (38 ATP/686 Kcal/mol)
Anabolic Reactions
All reactions in a cell that build new molecules are known as anabolic reactions.
EX:
6CO2 + 6H2O + Light energy (686 Kcal/mol) ⎯⎯→ C6H12O6 + 6O2
 Tungsten. (Вольфрам)
Tungsten. (Вольфрам) Құймалар. Механикалық қоспа
Құймалар. Механикалық қоспа Титан. Хром
Титан. Хром Теория электролитической диссоциации
Теория электролитической диссоциации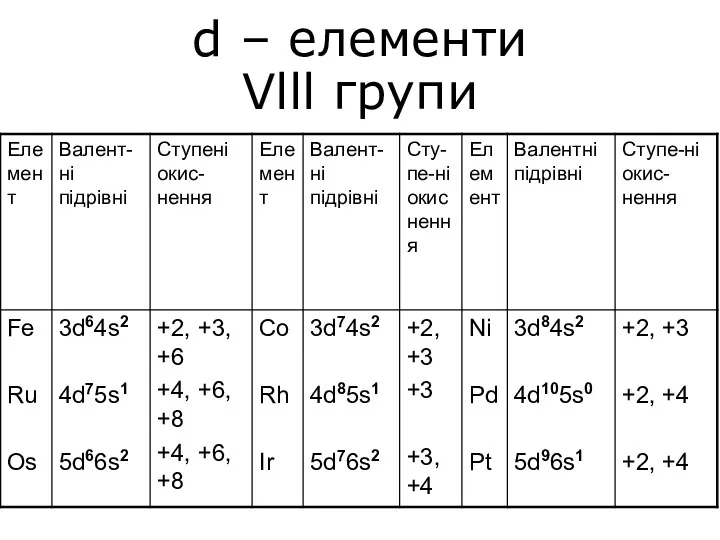 d – елементи Vlll групи
d – елементи Vlll групи Строение, реакционные способности и методы синтеза алкадиенов
Строение, реакционные способности и методы синтеза алкадиенов Ферменттер. Зерттелу тарихы
Ферменттер. Зерттелу тарихы Одноатомные и многоатомные спирты
Одноатомные и многоатомные спирты Огнетушащие средства и механизмы прекращения горения ими. Тема-8
Огнетушащие средства и механизмы прекращения горения ими. Тема-8 Розчини високомолекулярних сполук
Розчини високомолекулярних сполук Ионное произведение воды. Водородный показатель
Ионное произведение воды. Водородный показатель Строение атома. Химия. 11 класс
Строение атома. Химия. 11 класс Карбоновые кислоты и их функциональные производные. Хроматографические методы исследования
Карбоновые кислоты и их функциональные производные. Хроматографические методы исследования Технология монокристаллов и особо чистых веществ
Технология монокристаллов и особо чистых веществ Молекулярная кулинария
Молекулярная кулинария Предмет аналитической химии и ее основные понятия
Предмет аналитической химии и ее основные понятия Минерал хромдиопсид. Месторождения
Минерал хромдиопсид. Месторождения Evolution of Isoconversional Methods
Evolution of Isoconversional Methods Алкины
Алкины Биохимические аспекты биотрансформации лекарственных веществ
Биохимические аспекты биотрансформации лекарственных веществ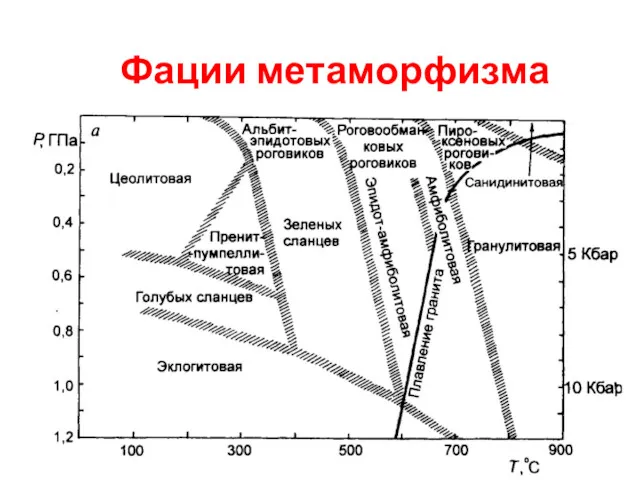 Фации метаморфизма
Фации метаморфизма Основные химические понятия и законы
Основные химические понятия и законы Закон сохранения массы веществ. Химические уравнения
Закон сохранения массы веществ. Химические уравнения Реакции ионного обмена 11 класс
Реакции ионного обмена 11 класс Топливо. Классификация
Топливо. Классификация Аммиак. 9 класс
Аммиак. 9 класс Номенклатура алканов
Номенклатура алканов Теоретические основы химической технологии переработки природных энергоносителей и углеводородных материалов
Теоретические основы химической технологии переработки природных энергоносителей и углеводородных материалов