Содержание
- 2. Outline Motivation Basic properties Crystal structure and phases Defects/Doping Solar cells Limiting factors Laboratory for Thin
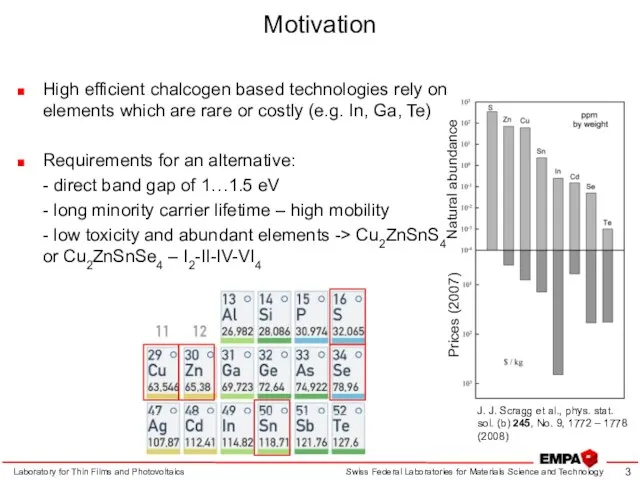
- 3. Motivation High efficient chalcogen based technologies rely on elements which are rare or costly (e.g. In,
- 4. Material properties Laboratory for Thin Films and Photovoltaics 1Chen et al., Crystal and electronic band structure
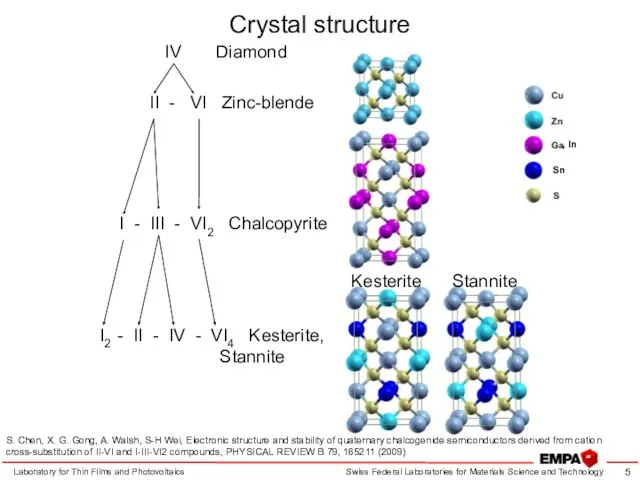
- 5. Crystal structure Laboratory for Thin Films and Photovoltaics S. Chen, X. G. Gong, A. Walsh, S-H
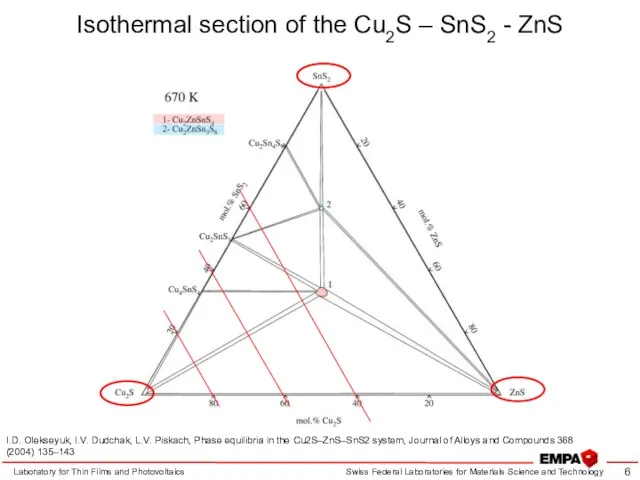
- 6. Isothermal section of the Cu2S – SnS2 - ZnS Laboratory for Thin Films and Photovoltaics I.D.
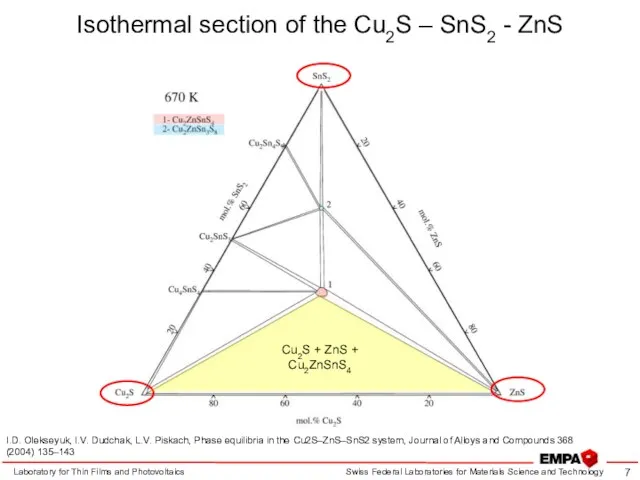
- 7. Isothermal section of the Cu2S – SnS2 - ZnS Laboratory for Thin Films and Photovoltaics I.D.
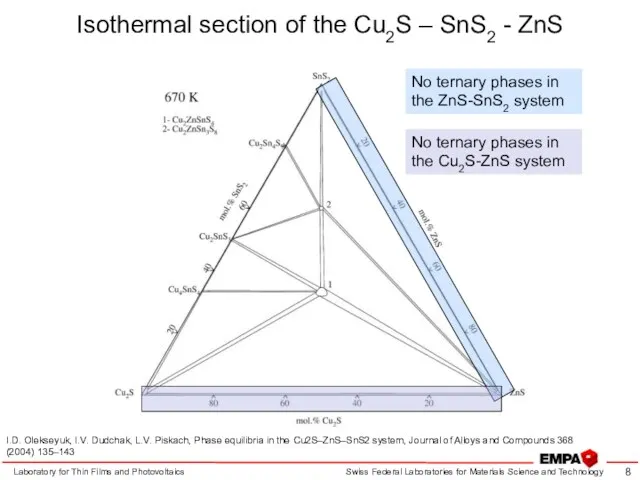
- 8. Isothermal section of the Cu2S – SnS2 - ZnS Laboratory for Thin Films and Photovoltaics I.D.
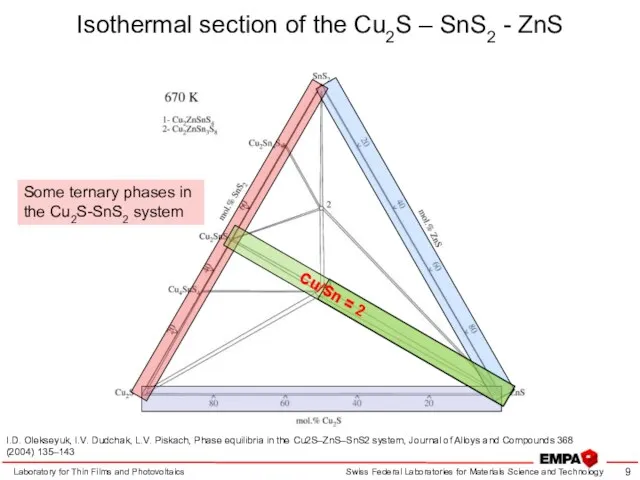
- 9. Isothermal section of the Cu2S – SnS2 - ZnS Laboratory for Thin Films and Photovoltaics I.D.
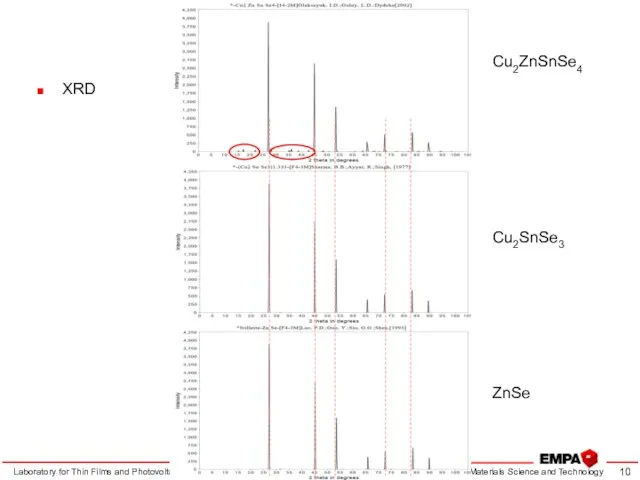
- 10. Kesterite characterization Laboratory for Thin Films and Photovoltaics XRD Cu2ZnSnSe4 Cu2SnSe3 ZnSe
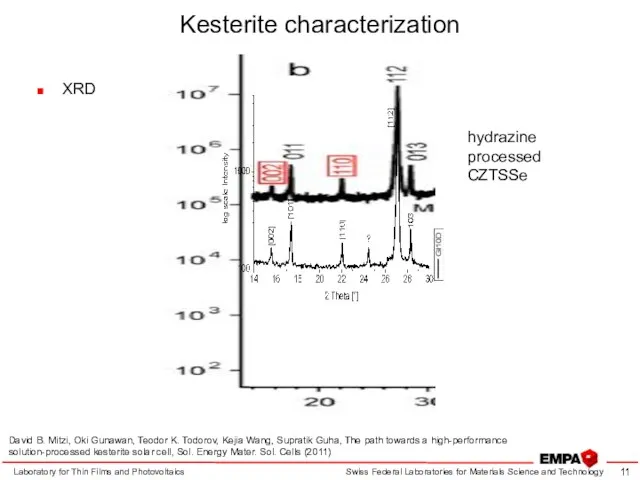
- 11. Kesterite characterization XRD Laboratory for Thin Films and Photovoltaics hydrazine processed CZTSSe David B. Mitzi, Oki
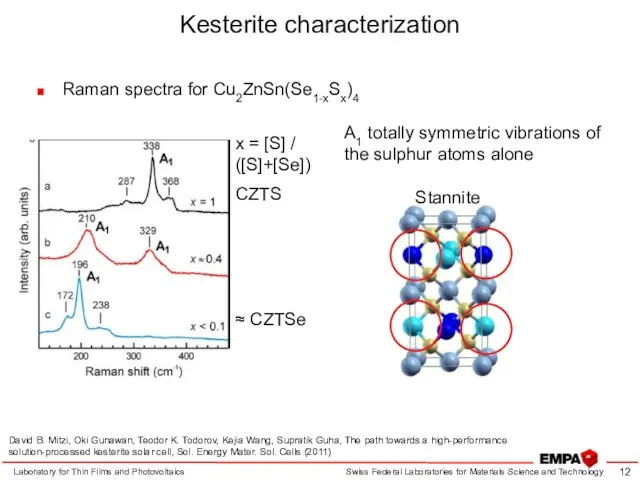
- 12. Kesterite characterization Raman spectra for Cu2ZnSn(Se1-xSx)4 Laboratory for Thin Films and Photovoltaics Stannite x = [S]
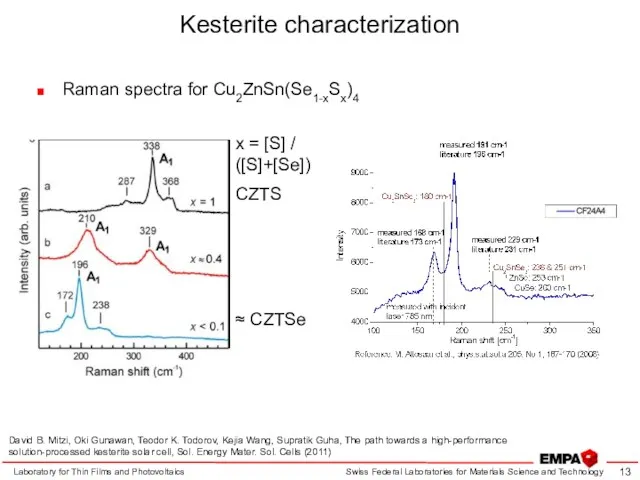
- 13. Kesterite characterization Raman spectra for Cu2ZnSn(Se1-xSx)4 Laboratory for Thin Films and Photovoltaics x = [S] /
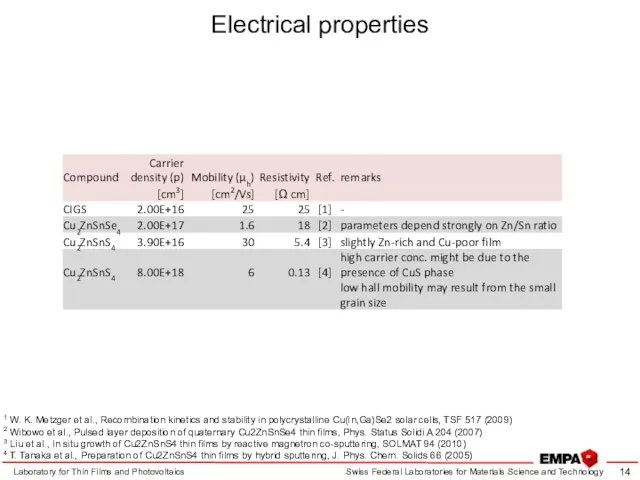
- 14. Electrical properties Laboratory for Thin Films and Photovoltaics 1 W. K. Metzger et al., Recombination kinetics
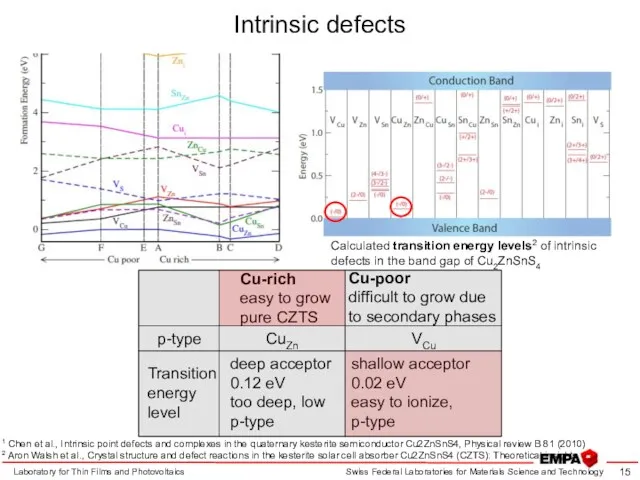
- 15. Intrinsic defects Laboratory for Thin Films and Photovoltaics 1 Chen et al., Intrinsic point defects and
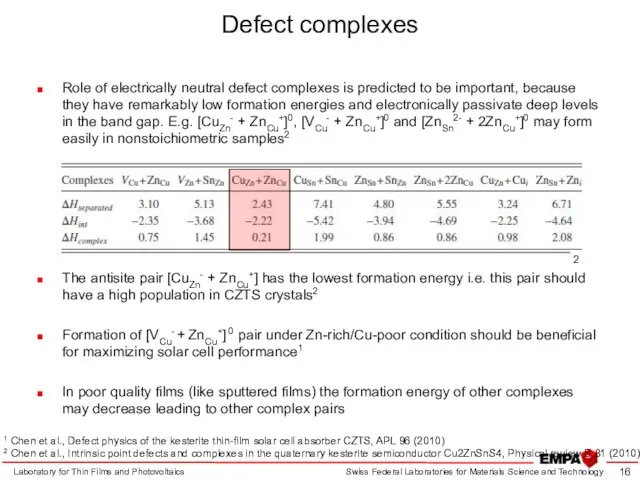
- 16. Defect complexes Role of electrically neutral defect complexes is predicted to be important, because they have
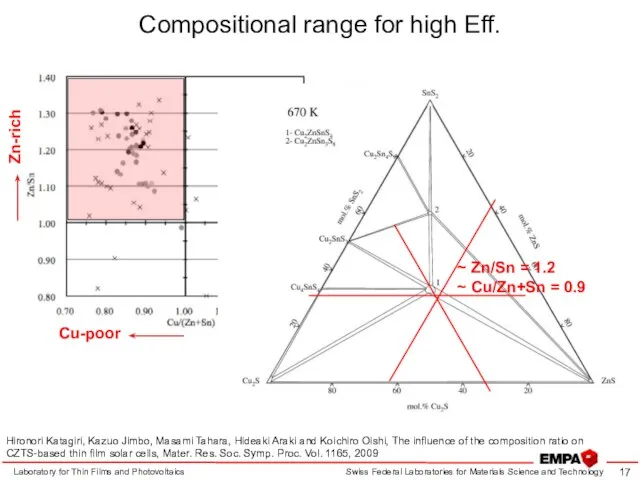
- 17. Laboratory for Thin Films and Photovoltaics Hironori Katagiri, Kazuo Jimbo, Masami Tahara, Hideaki Araki and Koichiro
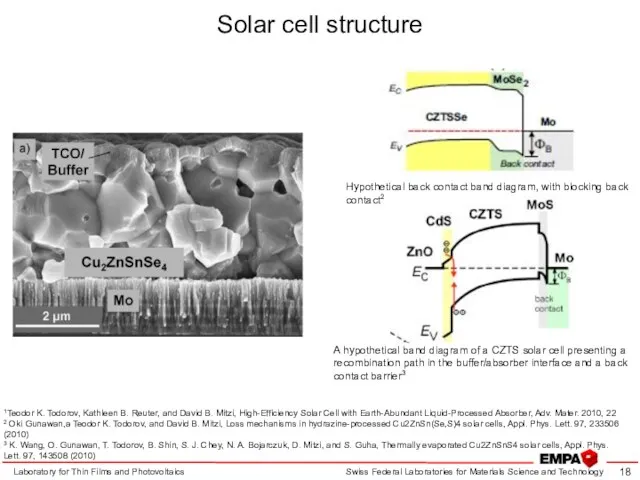
- 18. Solar cell structure Laboratory for Thin Films and Photovoltaics 1Teodor K. Todorov, Kathleen B. Reuter, and
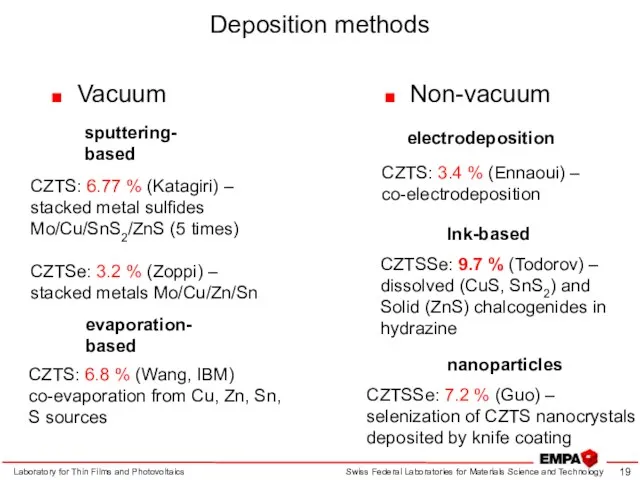
- 19. Deposition methods Vacuum Laboratory for Thin Films and Photovoltaics Non-vacuum sputtering- based evaporation- based CZTS: 6.77
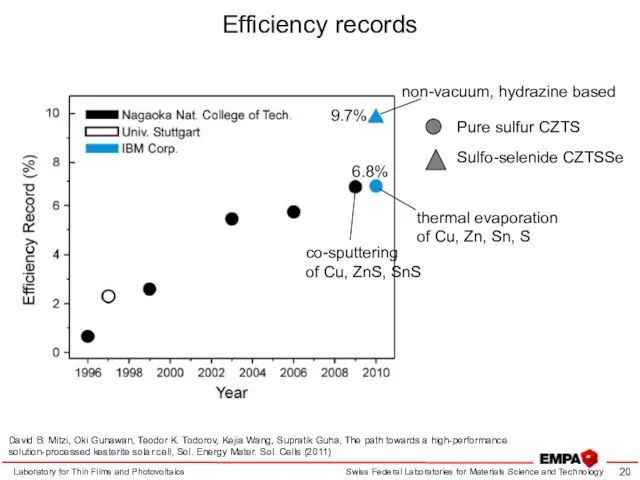
- 20. Efficiency records Laboratory for Thin Films and Photovoltaics David B. Mitzi, Oki Gunawan, Teodor K. Todorov,
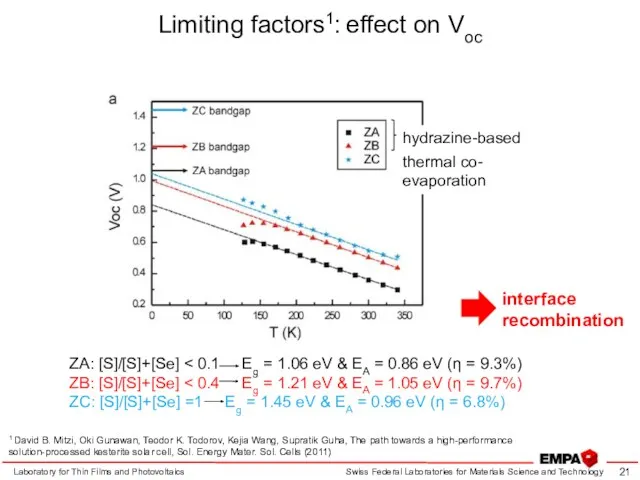
- 21. Limiting factors1: effect on Voc Laboratory for Thin Films and Photovoltaics 1 David B. Mitzi, Oki
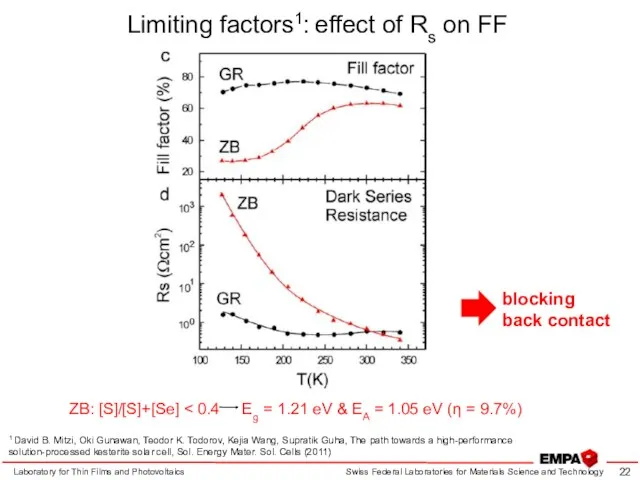
- 22. Limiting factors1: effect of Rs on FF Laboratory for Thin Films and Photovoltaics 1 David B.
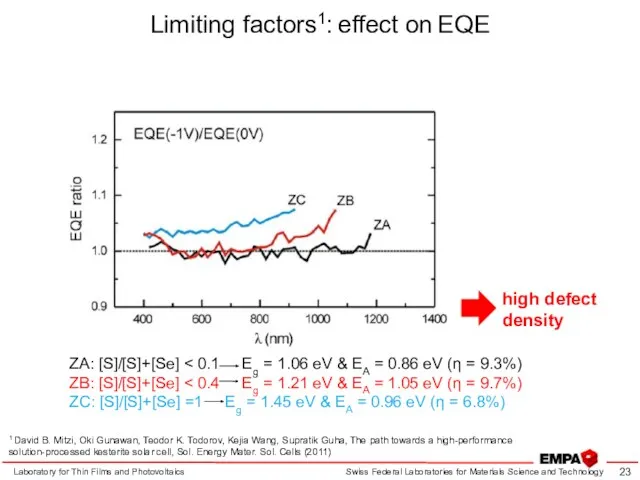
- 23. Limiting factors1: effect on EQE Laboratory for Thin Films and Photovoltaics 1 David B. Mitzi, Oki
- 24. Conclusions Formation and identification of parasitic phases (Cu2SnS3, Cu4SnS4, ZnS) Metal ratio control: Cu-poor / Zn-rich
- 25. Thank you for your attention ! Laboratory for Thin Films and Photovoltaics
- 26. Back up sildes Laboratory for Thin Films and Photovoltaics
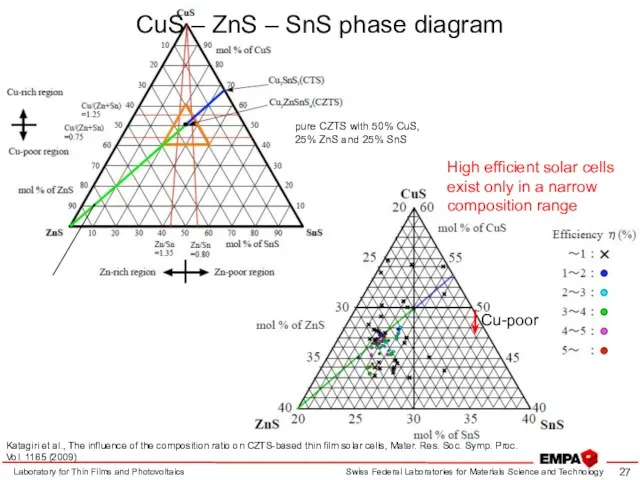
- 27. Laboratory for Thin Films and Photovoltaics Cu-poor pure CZTS with 50% CuS, 25% ZnS and 25%
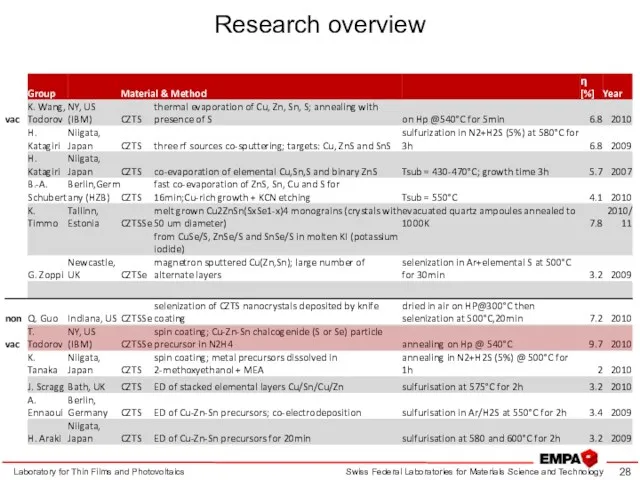
- 28. Research overview Laboratory for Thin Films and Photovoltaics
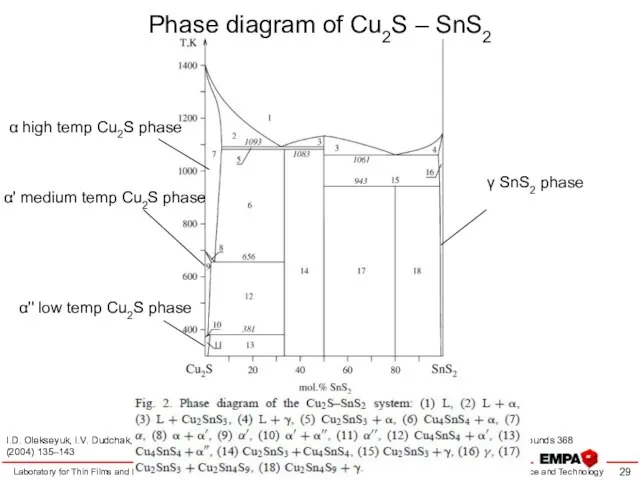
- 29. Phase diagram of Cu2S – SnS2 Laboratory for Thin Films and Photovoltaics I.D. Olekseyuk, I.V. Dudchak,
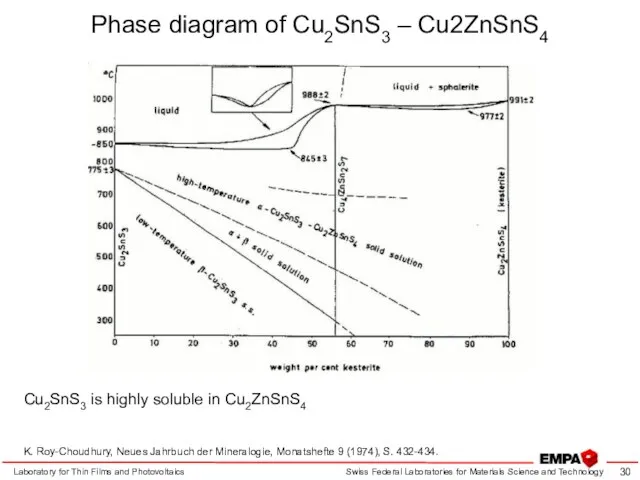
- 30. Laboratory for Thin Films and Photovoltaics K. Roy-Choudhury, Neues Jahrbuch der Mineralogie, Monatshefte 9 (1974), S.
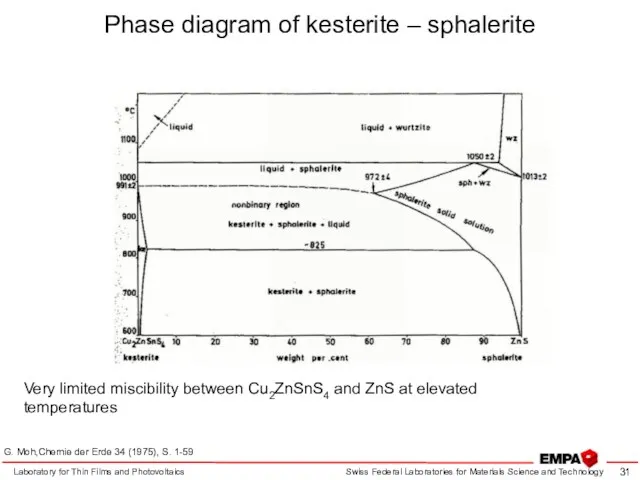
- 31. Laboratory for Thin Films and Photovoltaics Phase diagram of kesterite – sphalerite G. Moh,Chemie der Erde
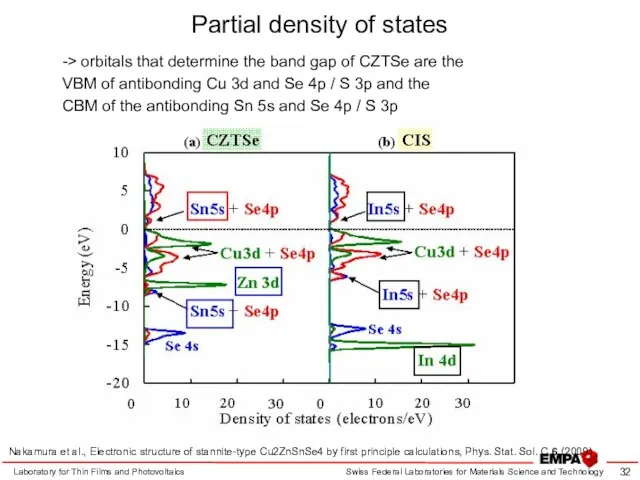
- 32. Partial density of states Laboratory for Thin Films and Photovoltaics -> orbitals that determine the band
- 34. Скачать презентацию


 Алкадиены. Непредельные углеводороды
Алкадиены. Непредельные углеводороды Классификация моторных масел
Классификация моторных масел Получение водорода. Проверка Н2 на чистоту. Практическая работа
Получение водорода. Проверка Н2 на чистоту. Практическая работа Симметрия в химии
Симметрия в химии Использование потенциостата-гальваностата Elins p-20x в электрохимических исследованиях
Использование потенциостата-гальваностата Elins p-20x в электрохимических исследованиях Переработка газа. Первичная переработка нефти. Лекция 9
Переработка газа. Первичная переработка нефти. Лекция 9 Титан және оның қорытпалары. Титаннан жасалған құралдар
Титан және оның қорытпалары. Титаннан жасалған құралдар Коллигативные свойства растворов
Коллигативные свойства растворов Окислительно-восстановительные реакции
Окислительно-восстановительные реакции Распространение пламени в газах
Распространение пламени в газах Молекулярно-кінетичні явища в дисперсних системах
Молекулярно-кінетичні явища в дисперсних системах Элементы химической термодинамики. 1 и 2 законы термодинамики. Химическое равновесие
Элементы химической термодинамики. 1 и 2 законы термодинамики. Химическое равновесие Роль хімії у житті суспільства
Роль хімії у житті суспільства Химическая связь
Химическая связь Карбоновые кислоты. (Лекция 6.3)
Карбоновые кислоты. (Лекция 6.3) Кристаллы и минералы
Кристаллы и минералы Получение полимеров из низкомолекулярных соединений
Получение полимеров из низкомолекулярных соединений Неон. Физические свойства
Неон. Физические свойства XXI ғасыр көшбасшысы
XXI ғасыр көшбасшысы Электрохимические процессы. Лекция 7
Электрохимические процессы. Лекция 7 Закономерности управления каталитическими процессами
Закономерности управления каталитическими процессами Кислород и его применение
Кислород и его применение Материаловедение. Контрольная работа
Материаловедение. Контрольная работа Умные полимеры в биотехнологии и медицине
Умные полимеры в биотехнологии и медицине Аммиак. 9 класс
Аммиак. 9 класс Гидролиз органических и неорганических соединений
Гидролиз органических и неорганических соединений Оксиды
Оксиды Основные сведения о строении атома
Основные сведения о строении атома