Содержание
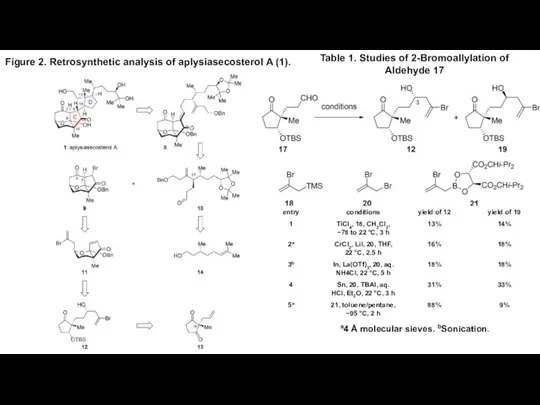
- 2. Figure 2. Retrosynthetic analysis of aplysiasecosterol A (1). Table 1. Studies of 2-Bromoallylation of Aldehyde 17
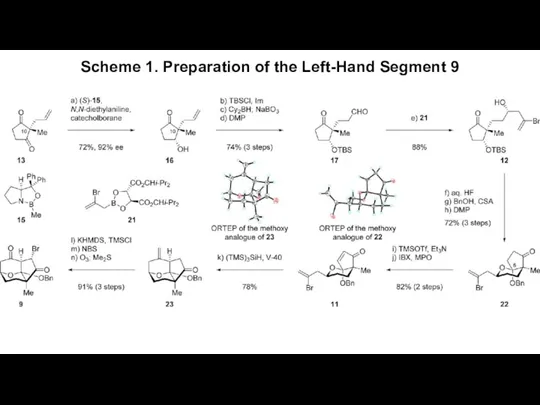
- 3. Scheme 1. Preparation of the Left-Hand Segment 9
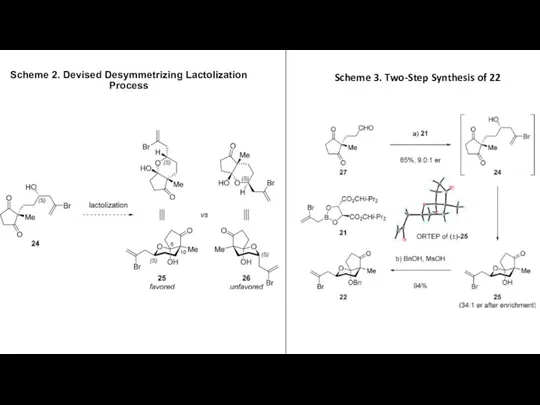
- 4. Scheme 2. Devised Desymmetrizing Lactolization Process Scheme 3. Two-Step Synthesis of 22
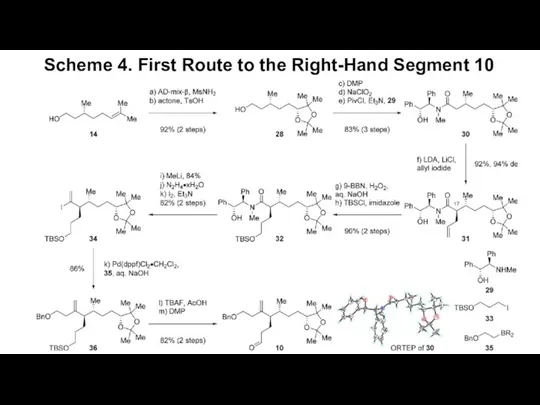
- 5. Scheme 4. First Route to the Right-Hand Segment 10
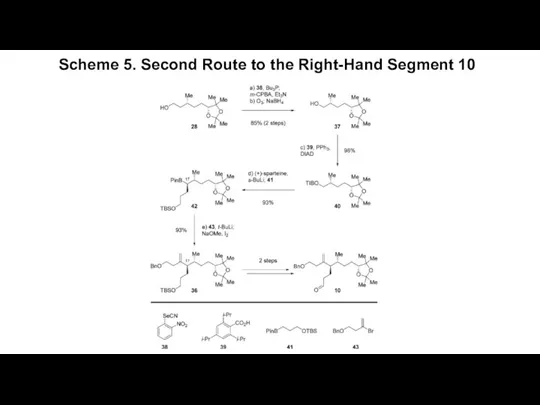
- 6. Scheme 5. Second Route to the Right-Hand Segment 10
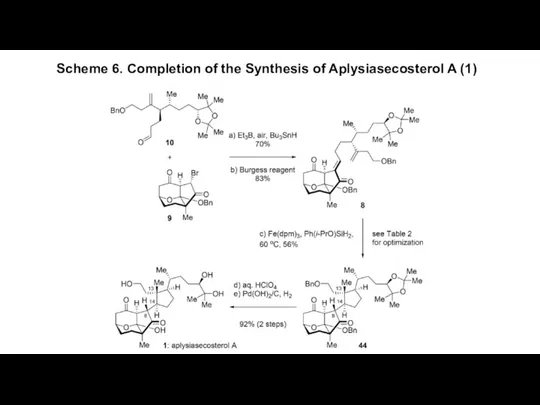
- 7. Scheme 6. Completion of the Synthesis of Aplysiasecosterol A (1)
- 8. Table 2. Conditions for the HAT Based Radical Cyclization of 8 aThe solvents were subjected to
- 10. Скачать презентацию

 Полистирол өндірісі
Полистирол өндірісі Амфотерные соединения
Амфотерные соединения Кислоты и основания. (Лекция 16)
Кислоты и основания. (Лекция 16) Классификация минералов
Классификация минералов Изотопы химических элементов
Изотопы химических элементов Свойства простых веществ в свете ОВР
Свойства простых веществ в свете ОВР Исследование процесса диффузии на примере движения частиц в жидкостях и газах
Исследование процесса диффузии на примере движения частиц в жидкостях и газах Азотная кислота и её соли
Азотная кислота и её соли Контроль результатов обучения химии
Контроль результатов обучения химии Фенол. Состав молекулы фенола
Фенол. Состав молекулы фенола Предмет органической химии
Предмет органической химии Искусственные и трансурановые элементы
Искусственные и трансурановые элементы Ртуть. Применение ртути и ее соединений
Ртуть. Применение ртути и ее соединений Золото. Виды золота
Золото. Виды золота Органические вещества: производные углеводородов
Органические вещества: производные углеводородов МЫШЬЯК
МЫШЬЯК Алюминий. Строение
Алюминий. Строение Магматические породы и постмагматические процессы
Магматические породы и постмагматические процессы Альдегиды и кетоны. 10 класс
Альдегиды и кетоны. 10 класс Происхождение названий минералов
Происхождение названий минералов Химическая посуда и лабораторное оборудование
Химическая посуда и лабораторное оборудование Валентность и степень окисления. Правила определения степеней окисления элементов
Валентность и степень окисления. Правила определения степеней окисления элементов Типы химических реакций
Типы химических реакций Водород. Общая характеристика, получение, свойства
Водород. Общая характеристика, получение, свойства Химические вещества и материалы в индустрии красоты. Номенклатура и классификация органических соединений
Химические вещества и материалы в индустрии красоты. Номенклатура и классификация органических соединений Спирты (алканолы)
Спирты (алканолы) Алкадиены (диеновые углеводороды)
Алкадиены (диеновые углеводороды)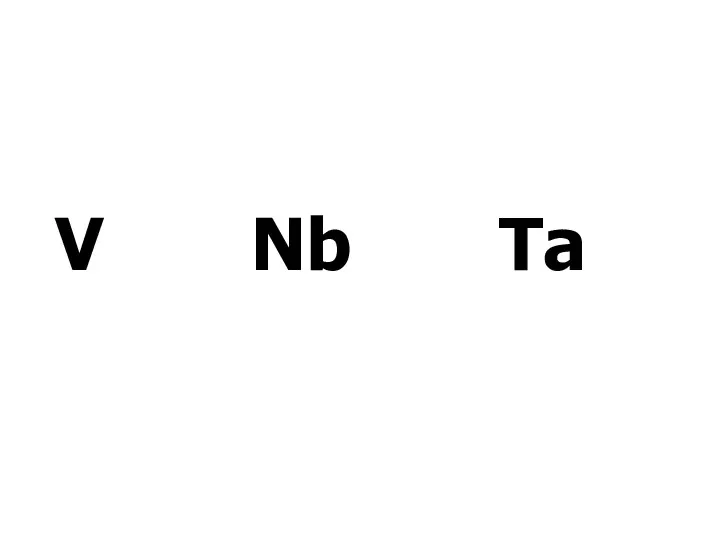 Метали V групи побічної підгрупи (V, Nb, Ta)
Метали V групи побічної підгрупи (V, Nb, Ta)