Содержание
- 2. A repeating pattern of chemical properties in elements is called periodicity. What is the periodicity?
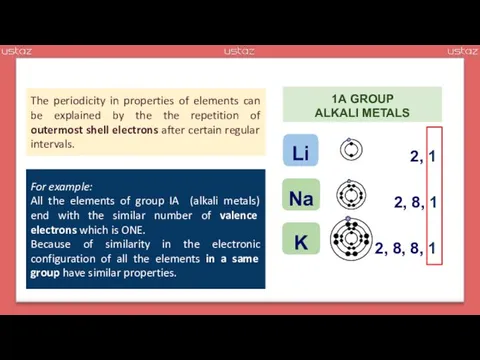
- 3. The periodicity in properties of elements can be explained by the the repetition of outermost shell
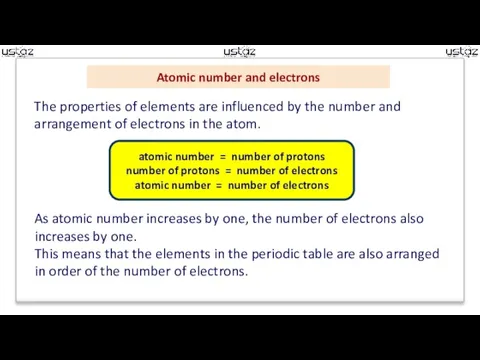
- 4. Atomic number and electrons The properties of elements are influenced by the number and arrangement of
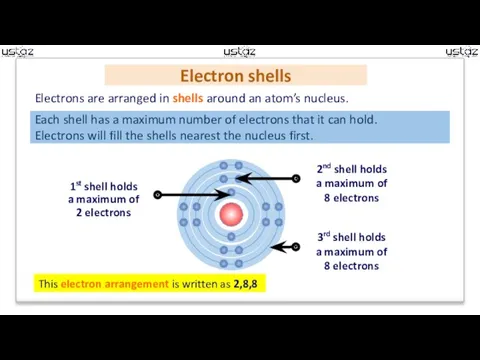
- 5. Electron shells Electrons are arranged in shells around an atom’s nucleus. This electron arrangement is written
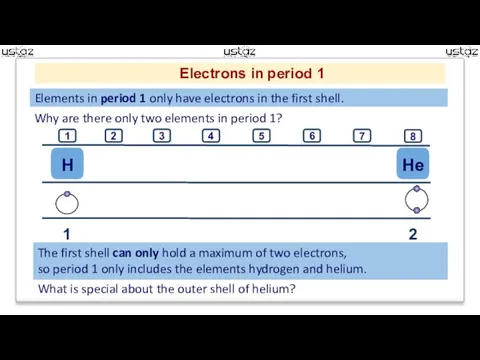
- 6. Electrons in period 1 Elements in period 1 only have electrons in the first shell. Why
- 7. Elements in period 2 all have a complete first shell. Аt second shell the number of
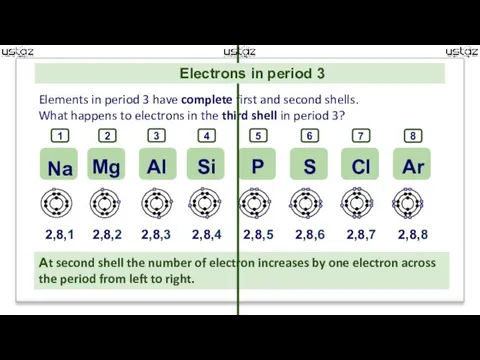
- 8. 2,8,1 2,8,2 2,8,3 2,8,4 2,8,5 2,8,6 2,8,7 2,8,8 Elements in period 3 have complete first and
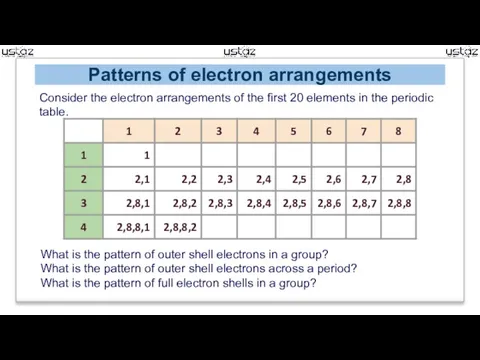
- 9. Patterns of electron arrangements Consider the electron arrangements of the first 20 elements in the periodic
- 10. Electron trends in the periodic table Trends down a group: By the start of new period
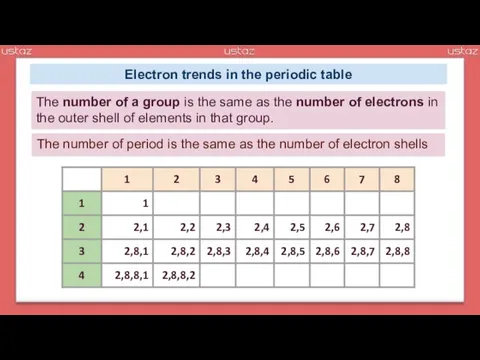
- 11. Electron trends in the periodic table The number of a group is the same as the
- 12. What is the electronic configuration?
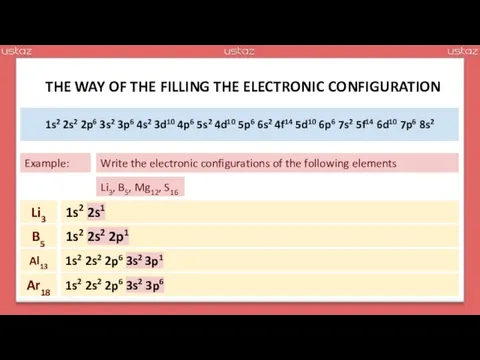
- 13. As you know, all electrons are distributed among the shells and subshells. The arrangement of electrons
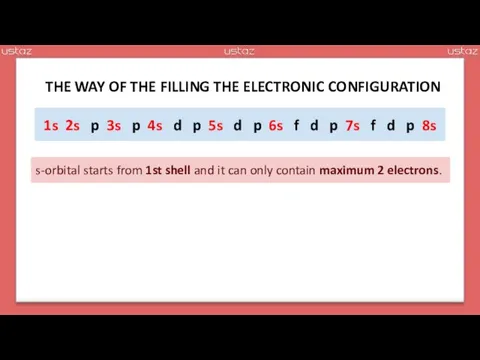
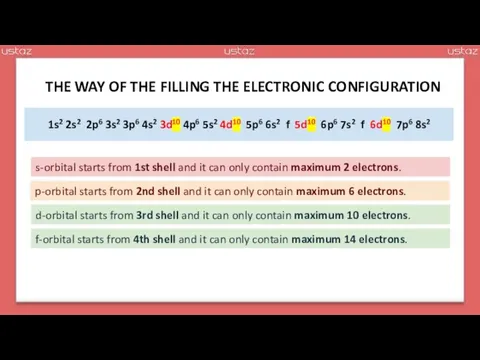
- 14. s s p s p s d p s d p s f d p s
- 15. s s p s p s d p s d p s f d p s
- 16. 1s 2s p 3s p 4s d p 5s d p 6s f d p 7s
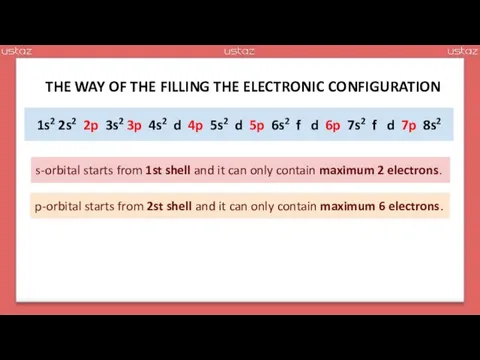
- 17. 1s2 2s2 p 3s2 p 4s2 d p 5s2 d p 6s2 f d p 7s2
- 18. 1s2 2s2 2p 3s2 3p 4s2 d 4p 5s2 d 5p 6s2 f d 6p 7s2
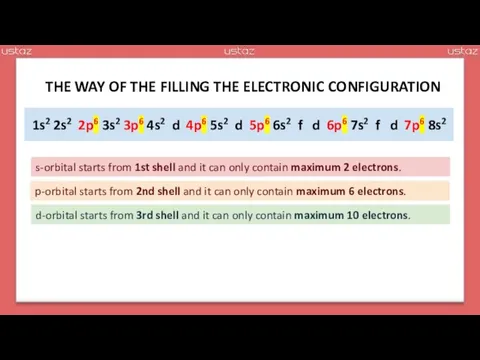
- 19. 1s2 2s2 2p6 3s2 3p6 4s2 d 4p6 5s2 d 5p6 6s2 f d 6p6 7s2
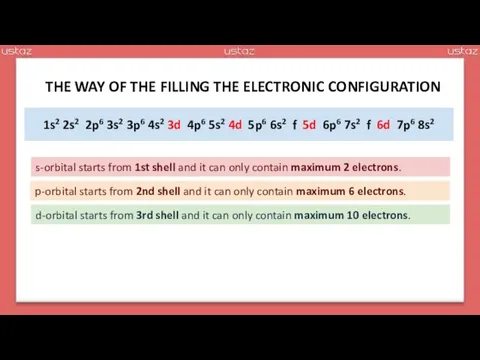
- 20. 1s2 2s2 2p6 3s2 3p6 4s2 3d 4p6 5s2 4d 5p6 6s2 f 5d 6p6 7s2
- 21. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 f 5d10 6p6 7s2
- 22. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f 5d10 6p6 7s2
- 23. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s2
- 24. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s2
- 25. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s2
- 26. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s2
- 27. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s2
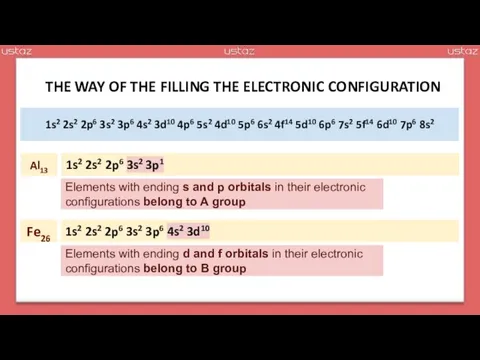
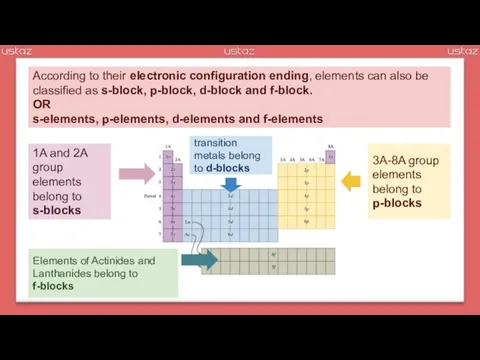
- 28. According to their electronic configuration ending, elements can also be classified as s-block, p-block, d-block and
- 29. Task 2. Work in pairs. Create the sentences from mixed-up words and share your answer with
- 30. Answer: Task 2 A repeating pattern of chemical properties in elements is called periodicity. All the
- 31. Task 3. Find the mistake. Here 4 sentences. In each sentences 2 words are changed their
- 32. Task 3. Find the mistake. Here 4 sentences. In each sentences 2 words are changed their
- 33. Task 4. Electron trends in the periodic table. Write 4 sentences and make best matching of
- 34. Task 4. Electron trends in the periodic table. Write 4 sentences and make best matching of
- 36. Скачать презентацию

















 Витаминдер. Витаминдердің классификациясы. Алиментарлы және екіншілік авитаминоздар. Гипервитаминоздар
Витаминдер. Витаминдердің классификациясы. Алиментарлы және екіншілік авитаминоздар. Гипервитаминоздар Генетическая связь между классами неорганических соединений. Урок 1
Генетическая связь между классами неорганических соединений. Урок 1 Электролитическая диссоциация. Протолитическая теория кислот и оснований. Лекция №5
Электролитическая диссоциация. Протолитическая теория кислот и оснований. Лекция №5 Кремний и его соединения
Кремний и его соединения Основные понятия и законы химии. Тема 1
Основные понятия и законы химии. Тема 1 Типы химических реакций. Тепловой эффект (11 класс)
Типы химических реакций. Тепловой эффект (11 класс) 20231104_prezentatsiya_teoriya_elektroliticheskoy_dissotsiatsii
20231104_prezentatsiya_teoriya_elektroliticheskoy_dissotsiatsii Икаит Ca[CO3]·6 (H2O)
Икаит Ca[CO3]·6 (H2O) Фосфаты и фосфонаты в стиральном порошке
Фосфаты и фосфонаты в стиральном порошке Электоролиз заңы
Электоролиз заңы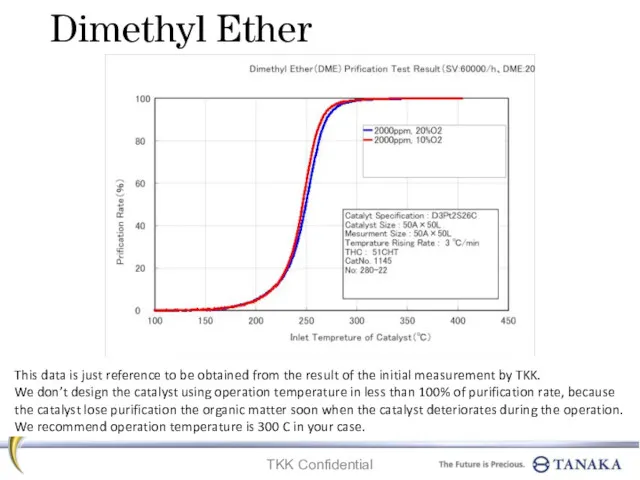 Dimethyl ether. Prification test result
Dimethyl ether. Prification test result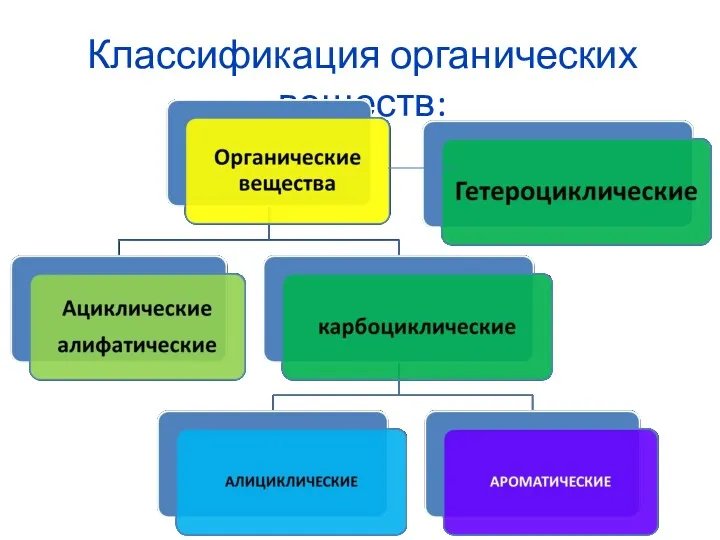 Классификация органических веществ
Классификация органических веществ Одноатомные спирты. Глицерин
Одноатомные спирты. Глицерин 20230204_ekzo_i_endo2_0
20230204_ekzo_i_endo2_0 Горение жидкого топлива
Горение жидкого топлива Равновесие в реакциях гидролиза. Лекция 6
Равновесие в реакциях гидролиза. Лекция 6 Аналитическая химия
Аналитическая химия Химические свойства алкенов
Химические свойства алкенов Вещества в твоей жизни
Вещества в твоей жизни Алкани
Алкани Коллоидные ПАВ. Мицеллообрaзование в растворах коллоидных ПАВ. Солюбилизация
Коллоидные ПАВ. Мицеллообрaзование в растворах коллоидных ПАВ. Солюбилизация Карбоновые кислоты и их гетерофункциональные производные
Карбоновые кислоты и их гетерофункциональные производные Чистые вещества и смеси
Чистые вещества и смеси Алкадиены: строение, номенклатура, гомологи, изомерия
Алкадиены: строение, номенклатура, гомологи, изомерия Алкины. Самостоятельная работа
Алкины. Самостоятельная работа Современные методы физико-химической биологии
Современные методы физико-химической биологии Альдегиды, свойства, получение, применение
Альдегиды, свойства, получение, применение Техника безопасности для учащихся в кабинете химии
Техника безопасности для учащихся в кабинете химии