Содержание
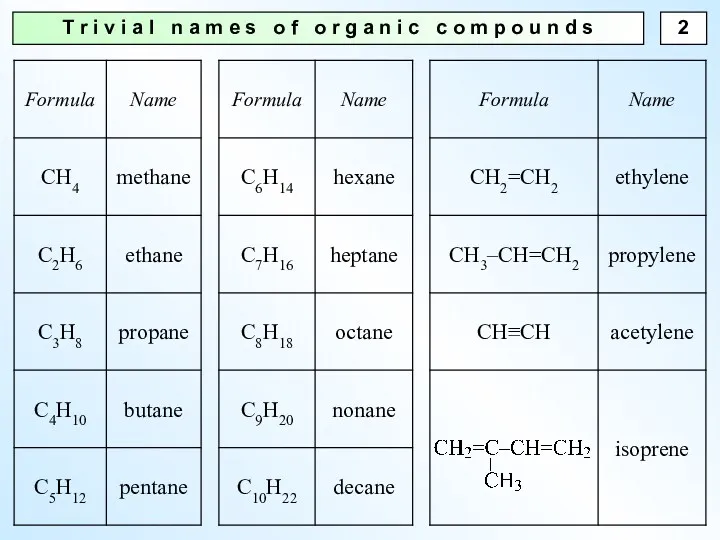
- 2. T r i v i a l n a m e s o f o r
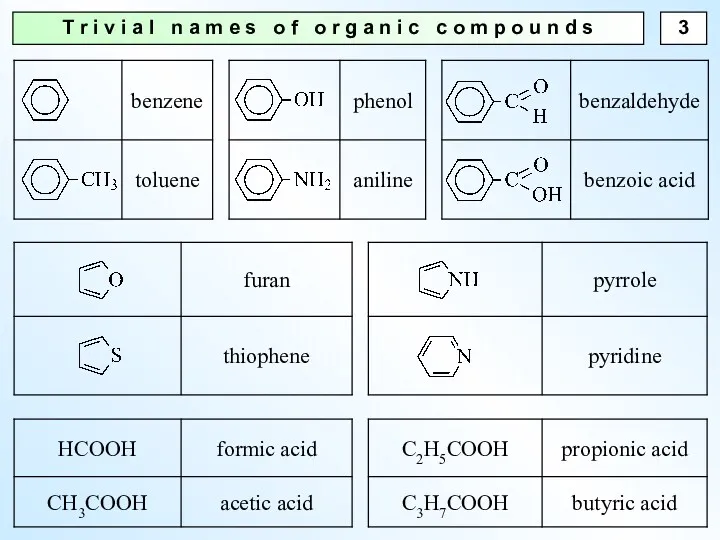
- 3. T r i v i a l n a m e s o f o r
- 4. R a d i c o f u n c t i o n a l
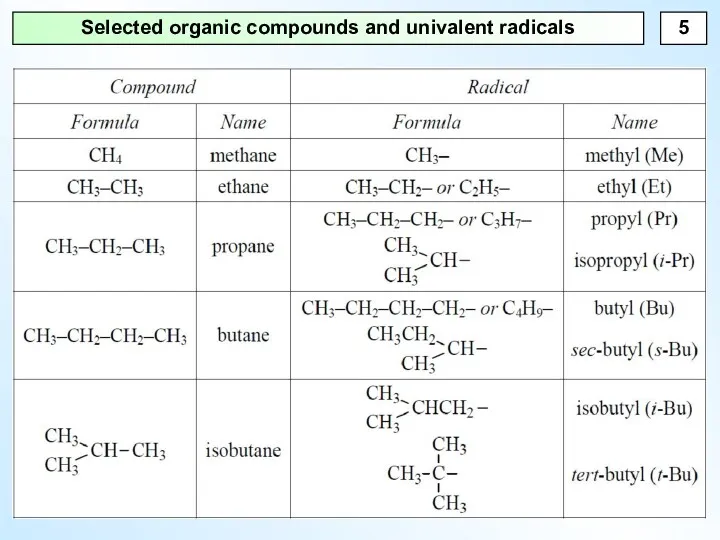
- 5. Selected organic compounds and univalent radicals
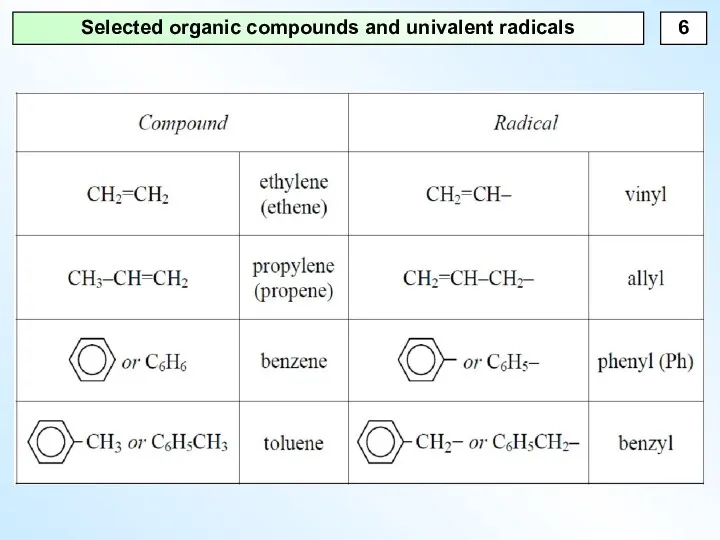
- 6. Selected organic compounds and univalent radicals
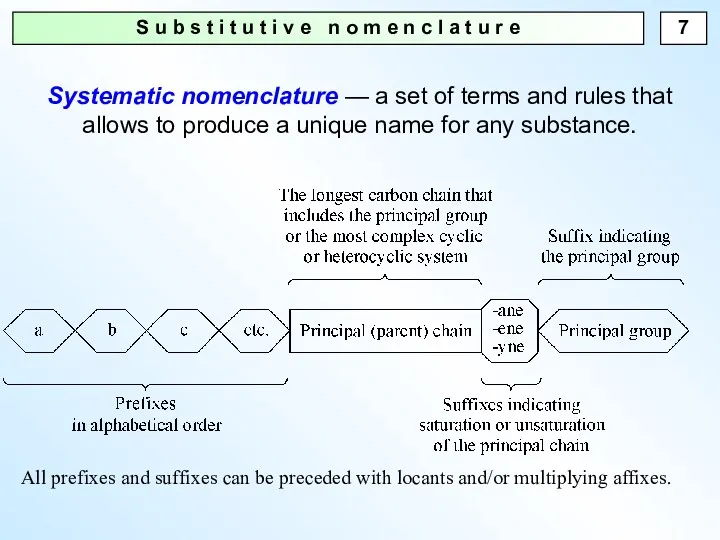
- 7. S u b s t i t u t i v e n o m e
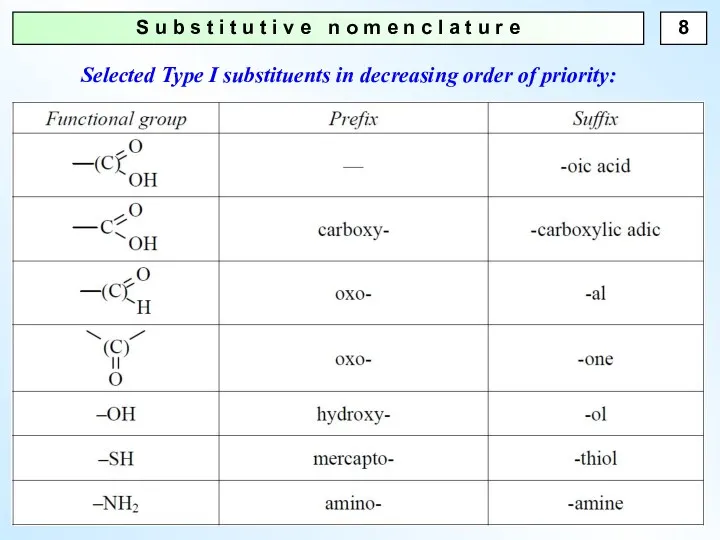
- 8. S u b s t i t u t i v e n o m e
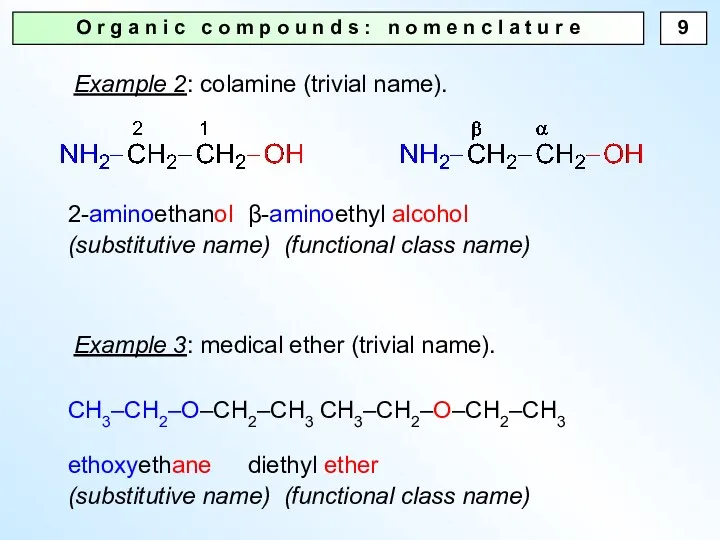
- 9. O r g a n i c c o m p o u n d s
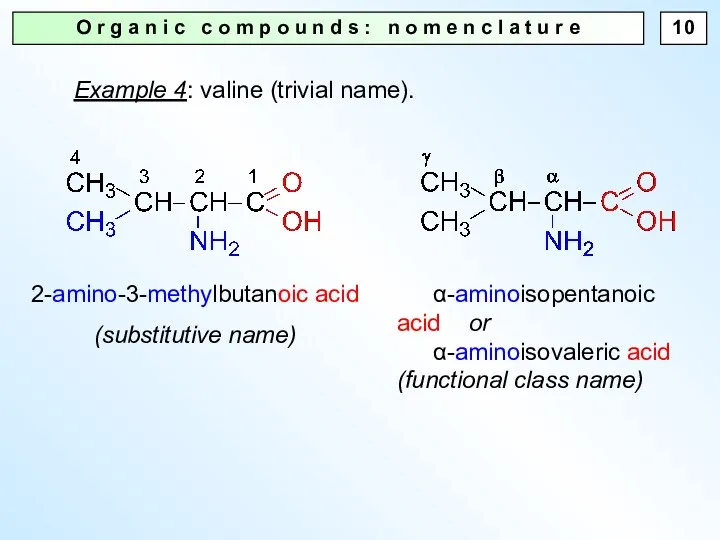
- 10. O r g a n i c c o m p o u n d s
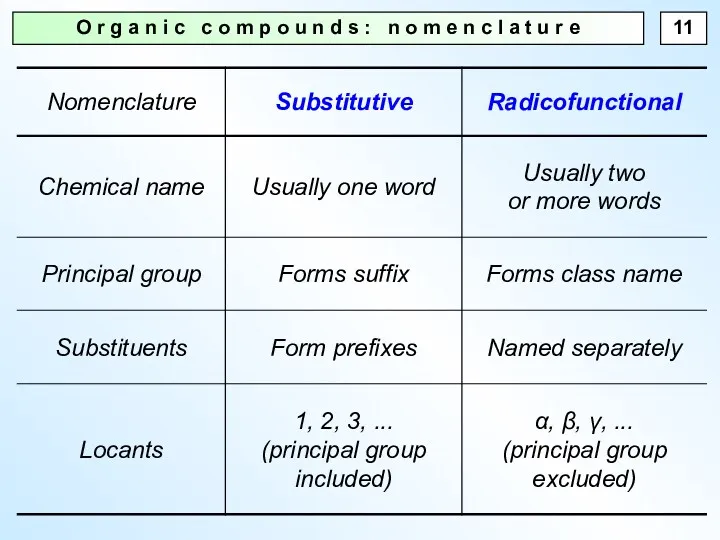
- 11. O r g a n i c c o m p o u n d s
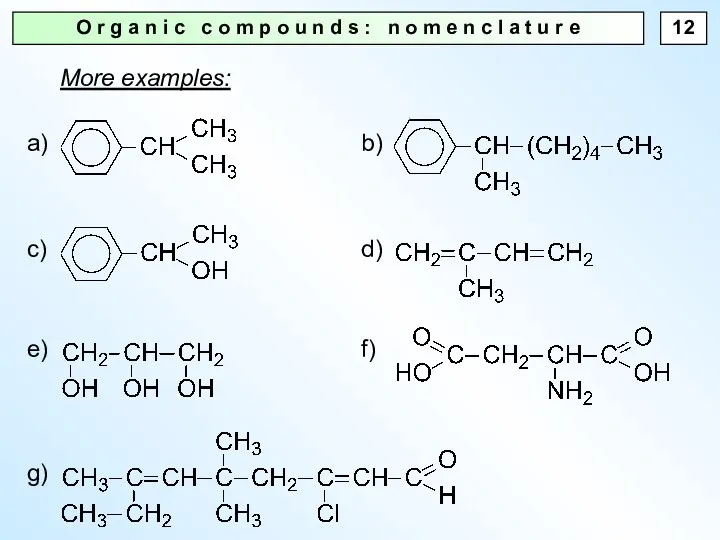
- 12. O r g a n i c c o m p o u n d s
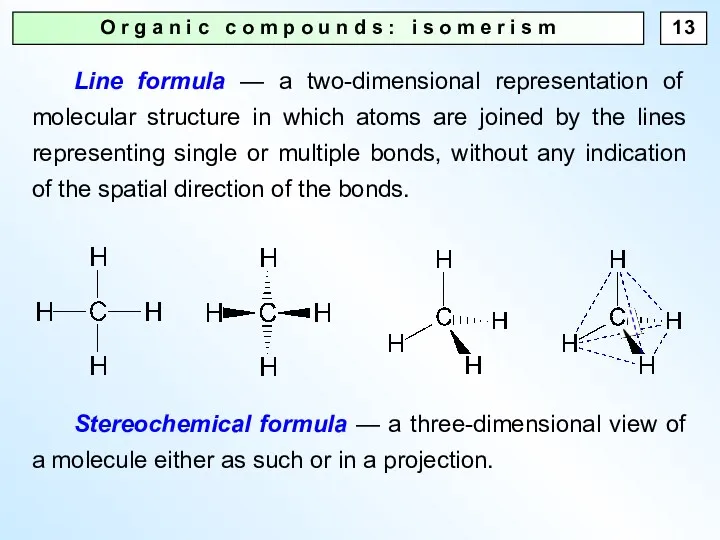
- 13. O r g a n i c c o m p o u n d s
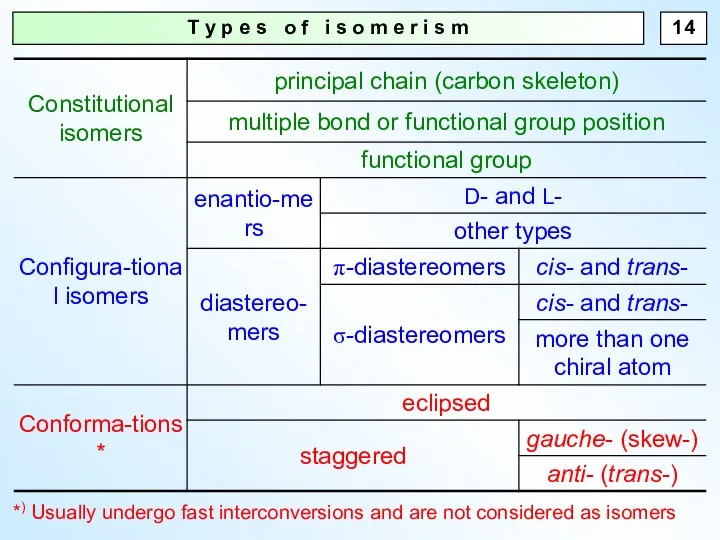
- 14. T y p e s o f i s o m e r i s m
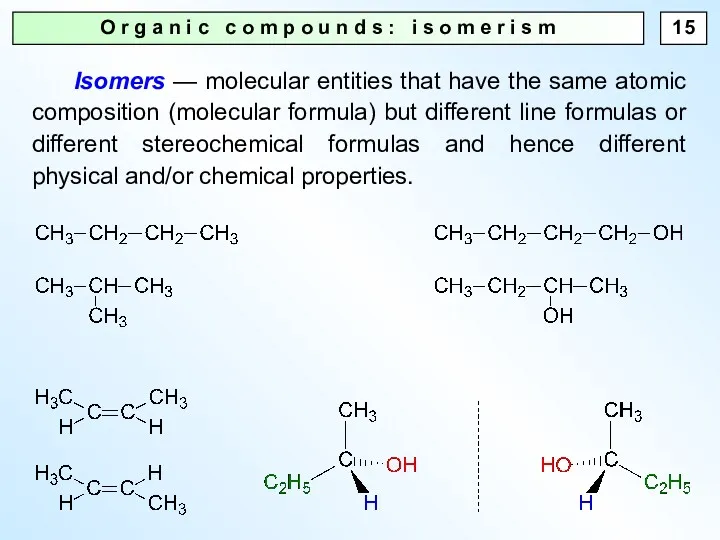
- 15. O r g a n i c c o m p o u n d s
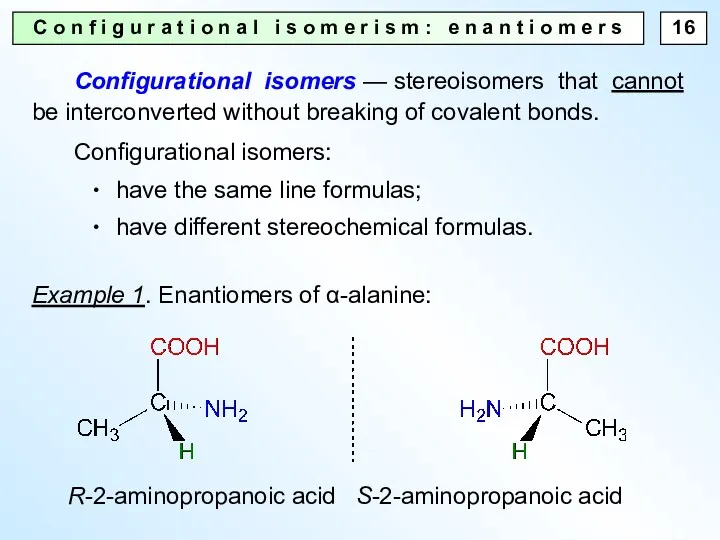
- 16. C o n f i g u r a t i o n a l i
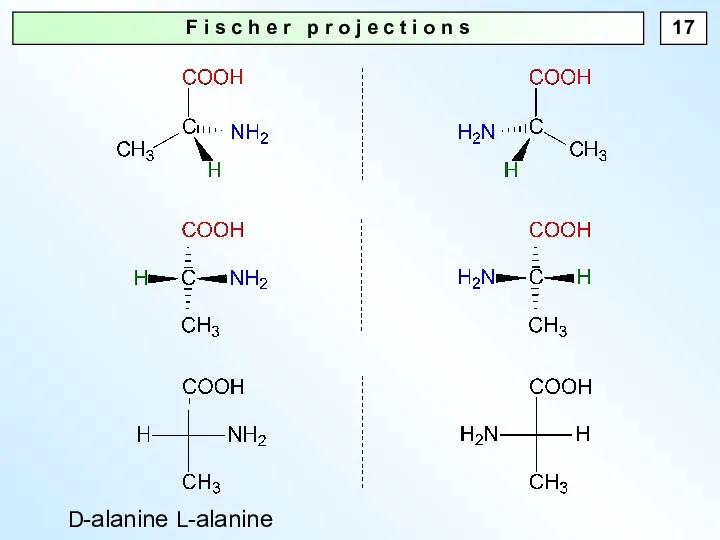
- 17. F i s c h e r p r o j e c t i o
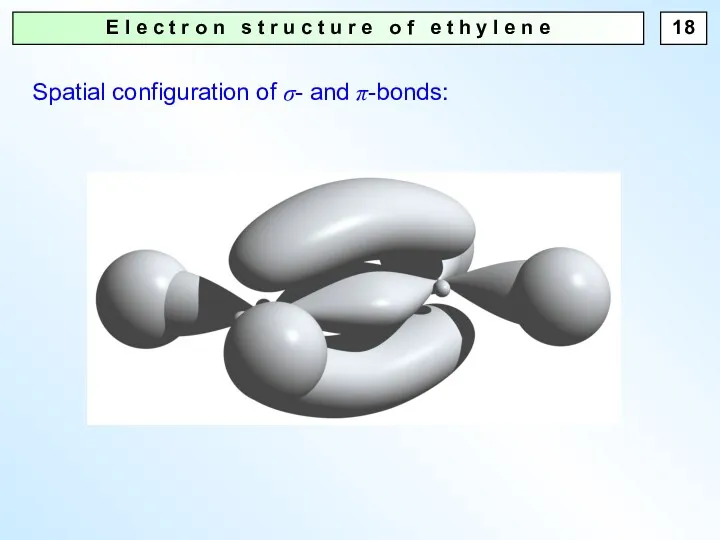
- 18. E l e c t r o n s t r u c t u r
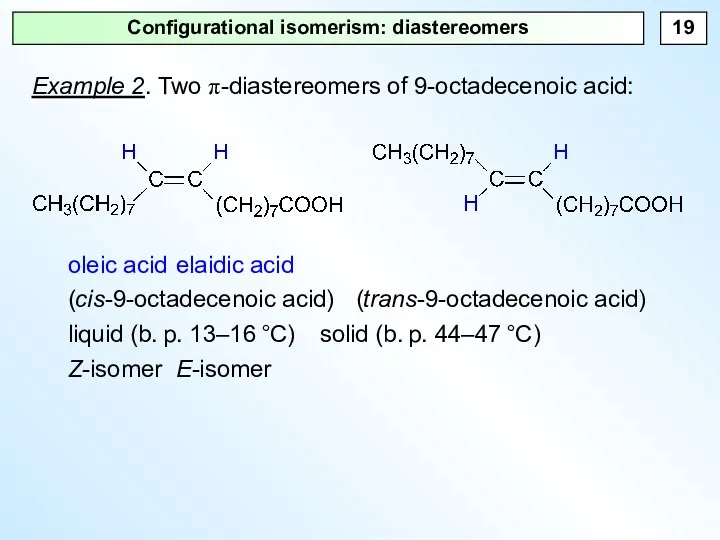
- 19. Configurational isomerism: diastereomers Example 2. Two π-diastereomers of 9-octadecenoic acid: oleic acid elaidic acid (cis-9-octadecenoic acid)
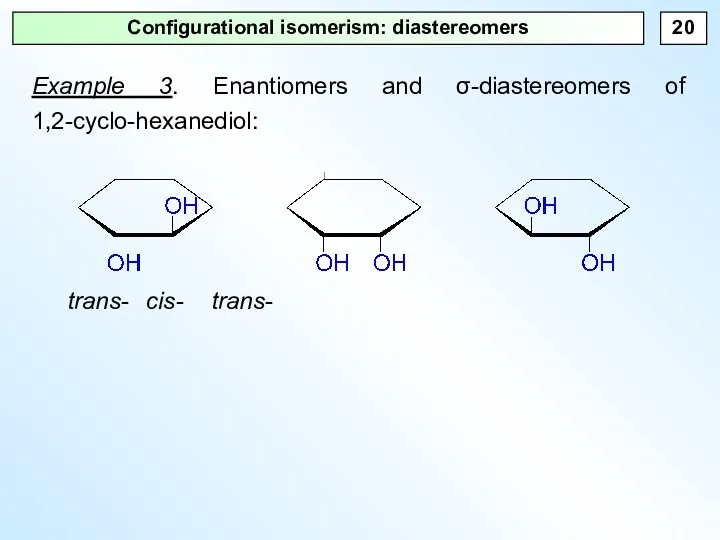
- 20. Configurational isomerism: diastereomers Example 3. Enantiomers and σ-diastereomers of 1,2-cyclo-hexanediol: trans- cis- trans-
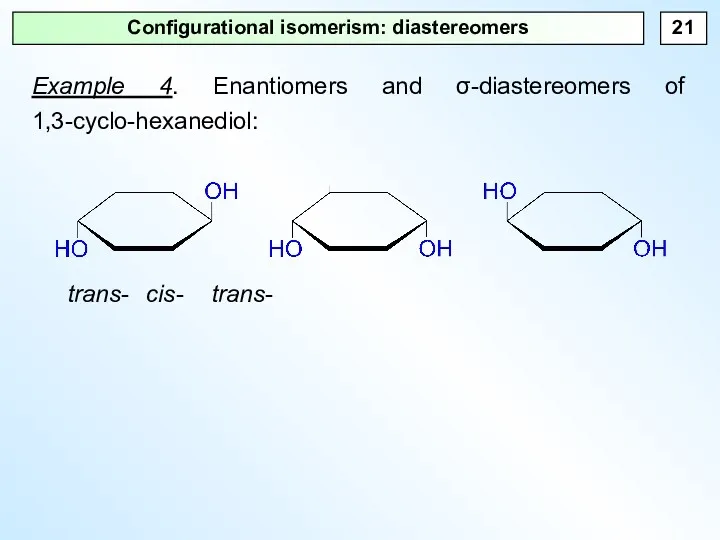
- 21. Configurational isomerism: diastereomers Example 4. Enantiomers and σ-diastereomers of 1,3-cyclo-hexanediol: trans- cis- trans-
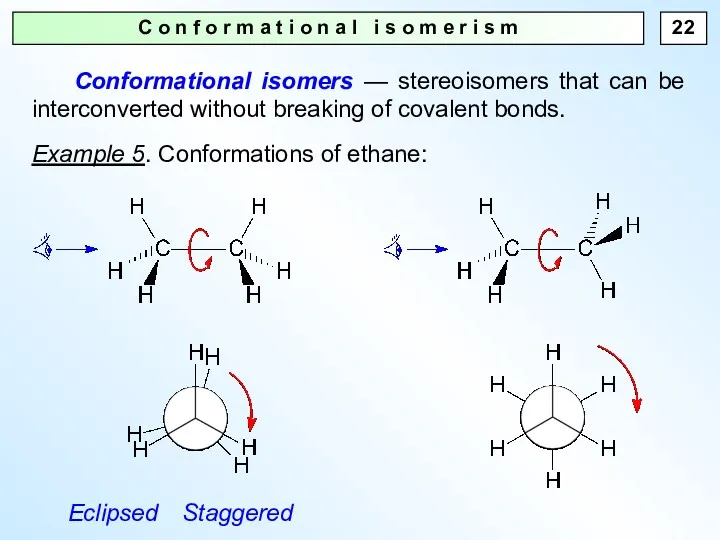
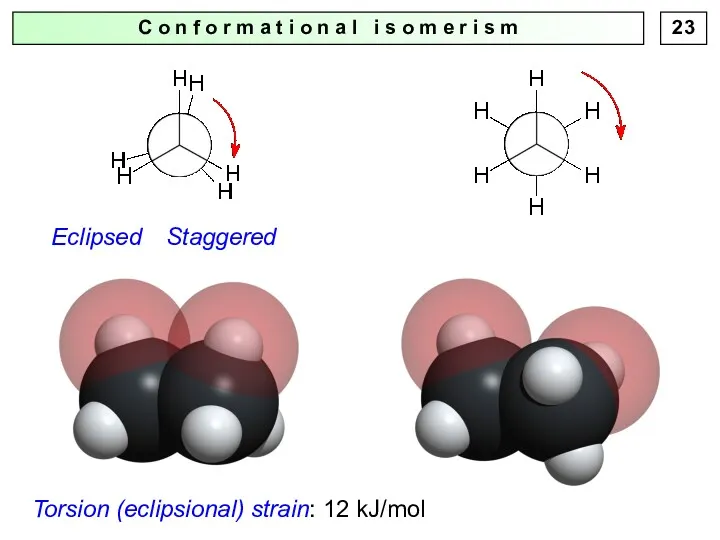
- 22. C o n f o r m a t i o n a l i s
- 23. C o n f o r m a t i o n a l i s
- 25. Скачать презентацию

 Процессы алкилирования
Процессы алкилирования Растворы
Растворы Серная кислота
Серная кислота Главная подгруппа VIII группы периодической системы. Девятнадцатая лекция
Главная подгруппа VIII группы периодической системы. Девятнадцатая лекция Органические вещества: производные углеводородов
Органические вещества: производные углеводородов Химическая связь. (Лекция 4, 5)
Химическая связь. (Лекция 4, 5) Дисахариди. Амінокислоти. Пептиди
Дисахариди. Амінокислоти. Пептиди Валентность и степень окисления элементов
Валентность и степень окисления элементов Выращивание кристаллов
Выращивание кристаллов яжелые металлы полезны или вредны?
яжелые металлы полезны или вредны? Характеристика элементов VIII-В группы. Семейство железа
Характеристика элементов VIII-В группы. Семейство железа Необоротні і оборотні хімічні процеси. Хімічна рівновага. Принцип Ле Шательє
Необоротні і оборотні хімічні процеси. Хімічна рівновага. Принцип Ле Шательє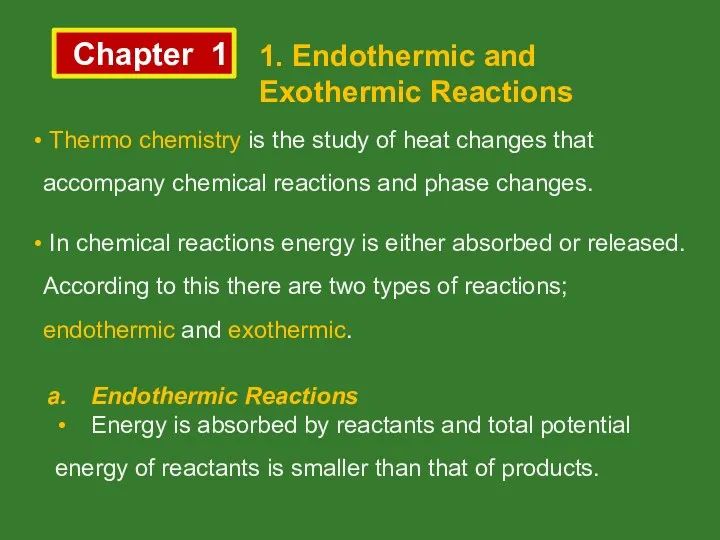 Chemical reactions and heat. (Chapter 1)
Chemical reactions and heat. (Chapter 1) Соединения химических элементов. 8 класс
Соединения химических элементов. 8 класс Окислительно-восстановительные реакции. Лабораторная работа
Окислительно-восстановительные реакции. Лабораторная работа Гидролиз неорганических солей
Гидролиз неорганических солей Період як особлива синтаксична конструкція
Період як особлива синтаксична конструкція Натуральные, искуственные и синтетические материалы
Натуральные, искуственные и синтетические материалы Природные источники углеводородов
Природные источники углеводородов Минералы группы кварца
Минералы группы кварца Сложные углеводы. Олигосахариды и полисахариды
Сложные углеводы. Олигосахариды и полисахариды Поверхностные явления. Адсорбция. Изотерма Ленгмюра
Поверхностные явления. Адсорбция. Изотерма Ленгмюра Флокуляційне очищення питної води за допомогою катіонних та аніонних флокулянтів
Флокуляційне очищення питної води за допомогою катіонних та аніонних флокулянтів Металлы II группы главной подгруппы
Металлы II группы главной подгруппы Арены. Бензол
Арены. Бензол Игра по химии В рамках периодической таблицы
Игра по химии В рамках периодической таблицы 20230306_gidroliz
20230306_gidroliz Углекислый газ
Углекислый газ