Содержание
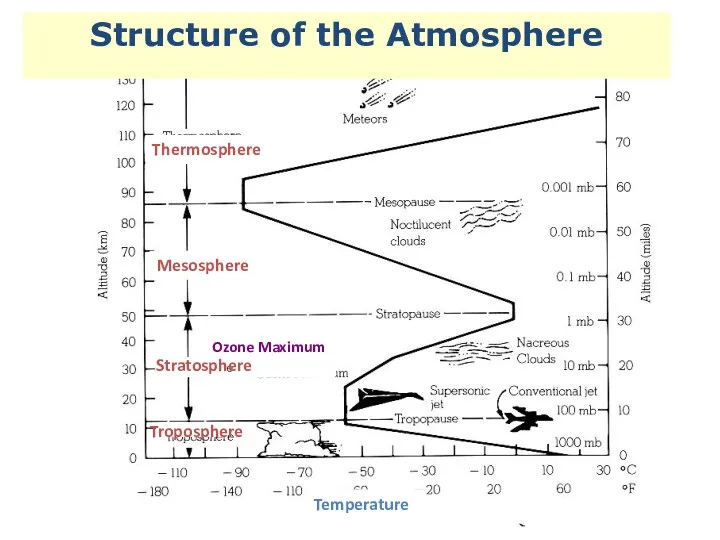
- 2. Structure of the Atmosphere Thermosphere Mesosphere Ozone Maximum Stratosphere Troposphere Temperature
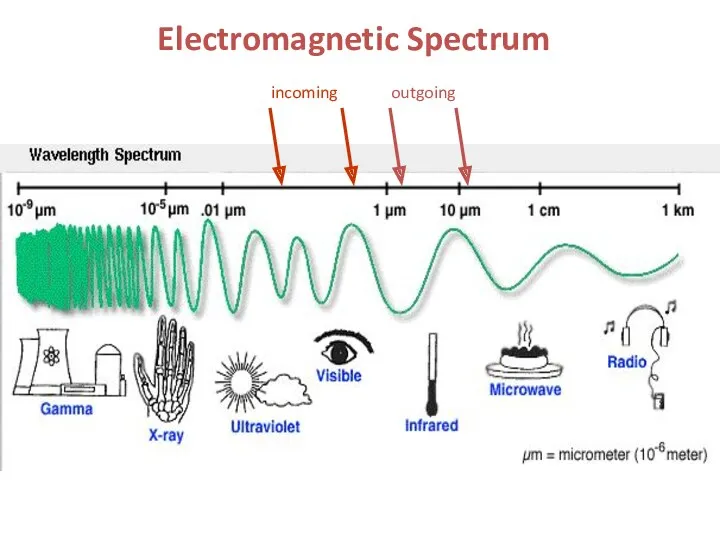
- 3. Electromagnetic Spectrum incoming outgoing
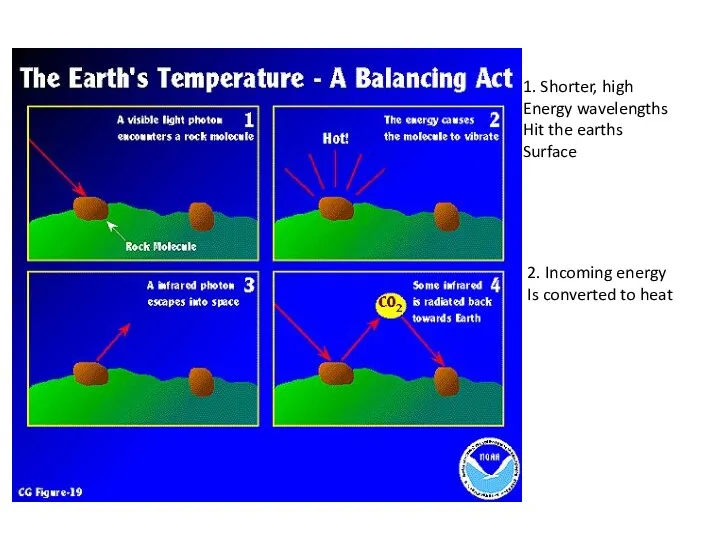
- 4. 1. Shorter, high Energy wavelengths Hit the earths Surface 2. Incoming energy Is converted to heat
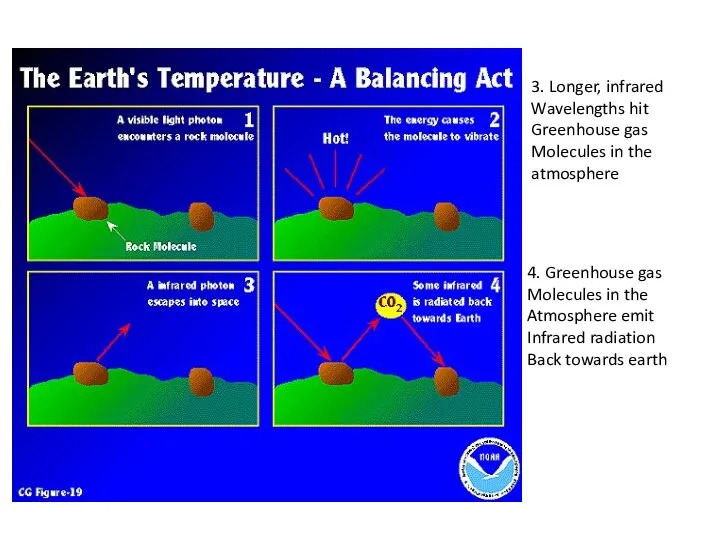
- 5. 3. Longer, infrared Wavelengths hit Greenhouse gas Molecules in the atmosphere 4. Greenhouse gas Molecules in
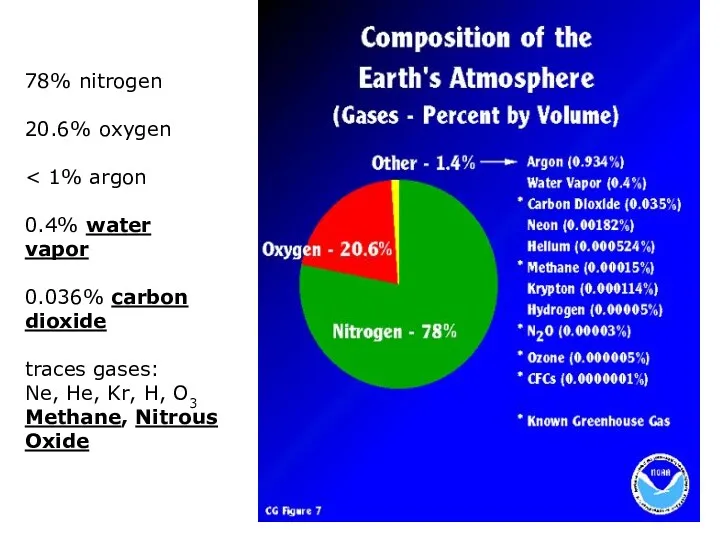
- 6. 78% nitrogen 20.6% oxygen 0.4% water vapor 0.036% carbon dioxide traces gases: Ne, He, Kr, H,
- 7. Absorption Spectra of Atmospheric Gases Anthes, p. 55 CH4 CO2 N2O H2O O2 & O3 atmosphere
- 8. Greenhouse gases absorb infrared radiation and prevent it from escaping to space. Carbon dioxide, methane, and
- 9. Climate Change - Greenhouse Gases To be an effective greenhouse gas, a molecule must: - absorb
- 10. Earth’s Atmospheric Gases Non- Greenhouse Gases 99% Greenhouse Gases 1%
- 11. Greenhouse Gases Carbon Dioxide Water Methane Nitrous Oxide
- 12. Greenhouse Gases Molecules must absorb light in the right regions - roughly 7 to 25 μm
- 13. - Greenhouse Gases Molecules absorbing light in the “IR window” regions are more effective Additional CO2
- 14. Selected Greenhouse Gases Carbon Dioxide (CO2) Source: Fossil fuel burning, deforestation Anthropogenic increase: 30% Average atmospheric
- 15. Greenhouse Effect & Global Warming The “greenhouse effect” & global warming are not the same thing.
- 16. Global Energy Redistribution
- 17. Radiation is not evenly distributed over the Surface of the earth. The northern latitudes have an
- 18. The climate engine II Since earth does rotate, air packets do not follow longitude lines (Coriolis
- 19. Atmospheric Pressure Decreases With Height Most of the energy is captured close to the surface That
- 20. Cloud effects Low clouds over ocean more clouds reflect heat (cooling) fewer clouds trap heat (warming)
- 21. Fig. 19-10, p. 513
- 22. - Greenhouse Gases H2O as a greenhouse gas - the molecule responsible for the most greenhouse
- 23. The sun plays a key role in the earth’s temperature Researchers think that atmospheric warming is
- 24. Water vapor is one of the most important elements of the climate system. A greenhouse gas,
- 25. Nitrogen (N) is an essential component of DNA, RNA, and proteins, the building blocks of life.
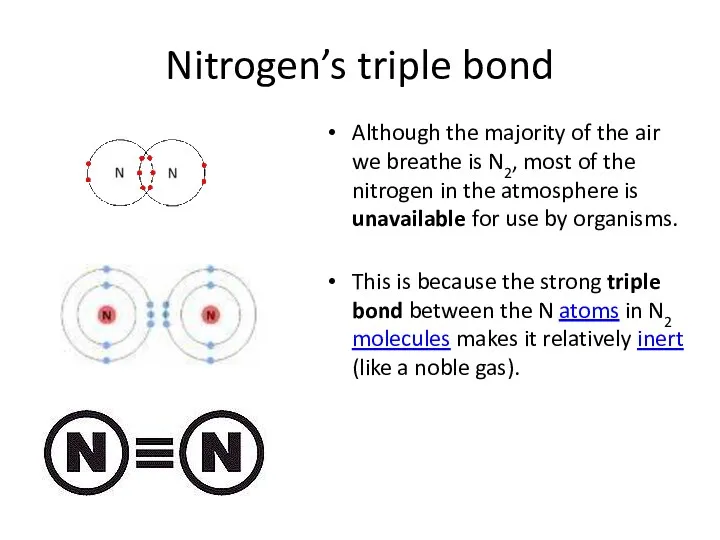
- 26. Nitrogen’s triple bond Although the majority of the air we breathe is N2, most of the
- 29. Forms of Nitrogen Urea ? CO(NH2)2 Ammonia ? NH3 (gaseous) Ammonium ? NH4 Nitrate ? NO3
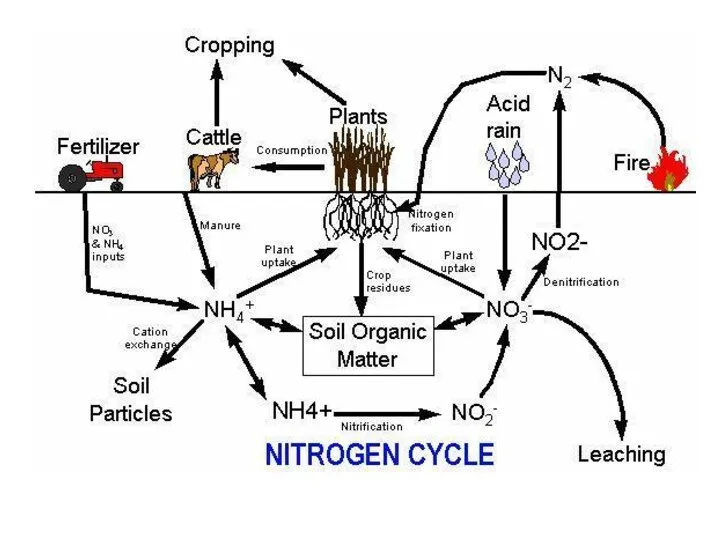
- 30. How can we use N2? In order for plants and animals to be able to use
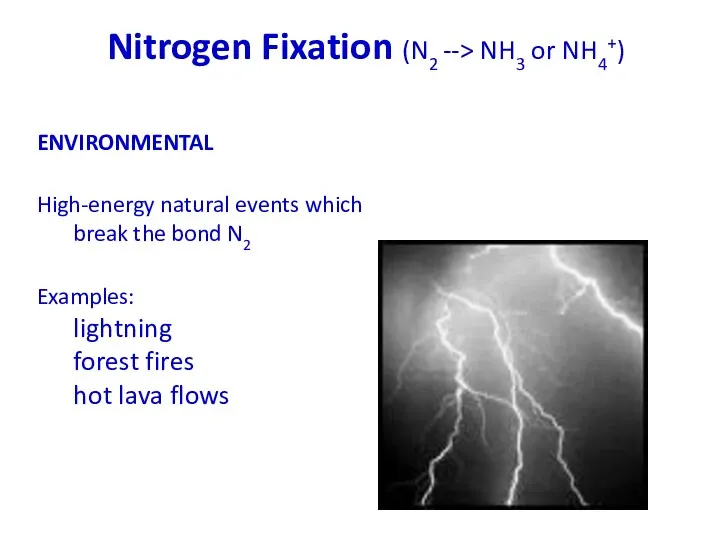
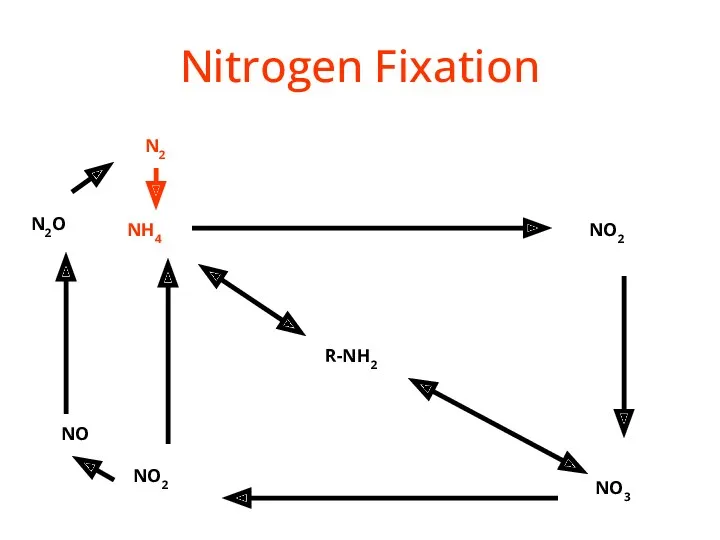
- 31. Nitrogen Fixation (N2 --> NH3 or NH4+) ENVIRONMENTAL High-energy natural events which break the bond N2
- 32. Nitrogen Fixation R-NH2 NH4 NO2 NO3 NO2 NO N2O N2
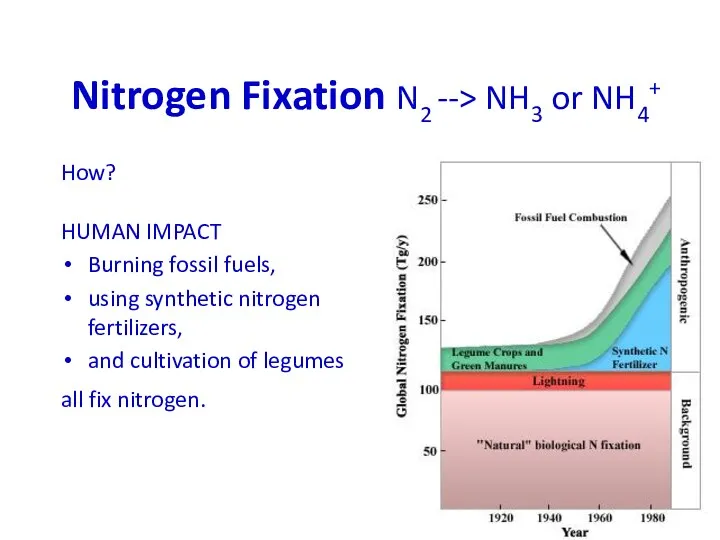
- 33. Nitrogen Fixation N2 --> NH3 or NH4+ How? HUMAN IMPACT Burning fossil fuels, using synthetic nitrogen
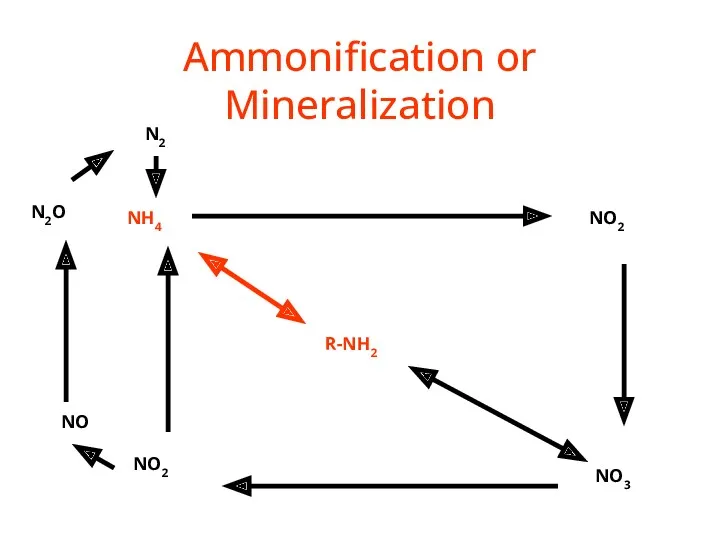
- 34. Ammonification or Mineralization R-NH2 NH4 NO2 NO3 NO2 NO N2O N2
- 35. Nitrogen Mineralization also called Ammonification Organic N --> NH4+ Decay of dead things, manure, etc. Done
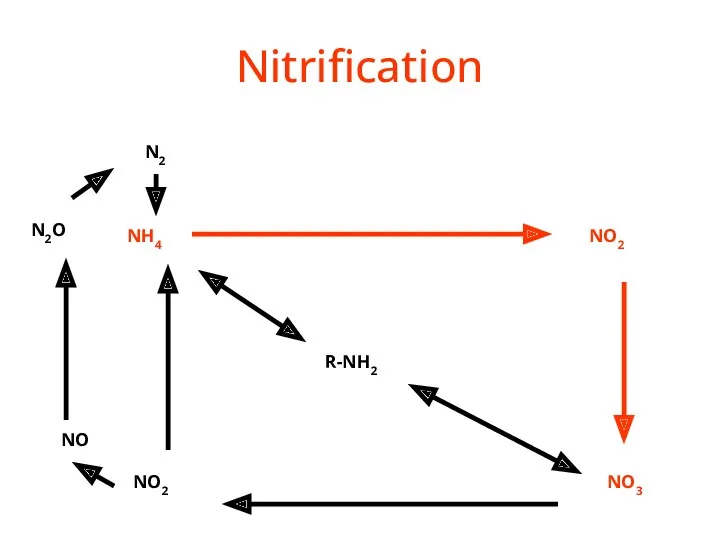
- 36. Nitrification R-NH2 NH4 NO2 NO3 NO2 NO N2O N2
- 37. Nitrification NH3 or NH4+ --> NO2- --> NO3- (Nitrifying) Bacteria add oxygen to nitrogen in two
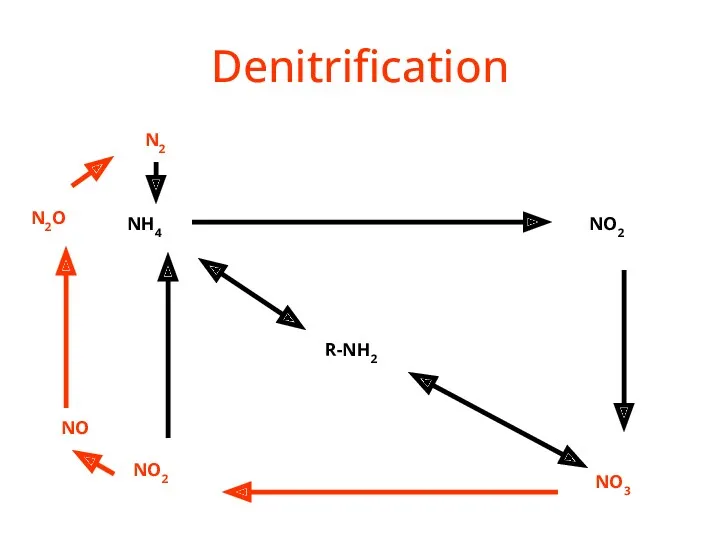
- 38. Denitrification R-NH2 NH4 NO2 NO3 NO2 NO N2O N2
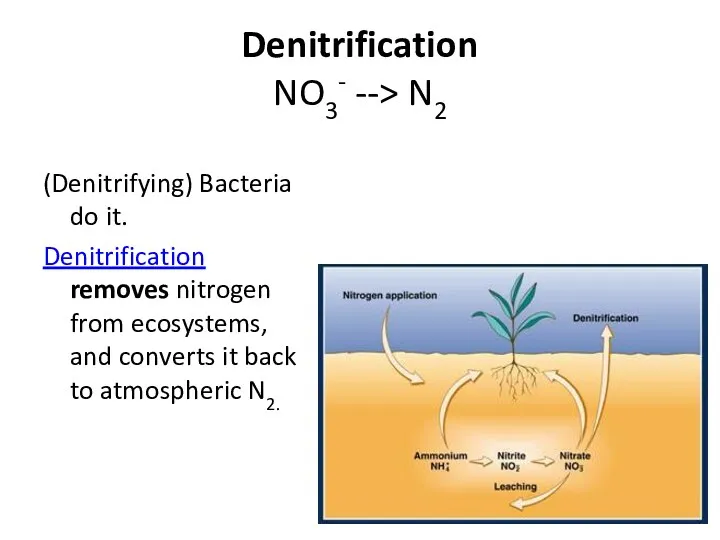
- 39. Denitrification NO3- --> N2 (Denitrifying) Bacteria do it. Denitrification removes nitrogen from ecosystems, and converts it
- 40. Denitrification Removes a limiting nutrient from the environment 4NO3- + C6H12O6? 2N2 + 6 H20 Inhibited
- 42. Nitrous oxide N2O Nitrous oxide, commonly known as laughing gas, nitrous, nitro, or NOS is a
- 43. N2O/O2 sedation It is necessary to use oxygen with nitrous oxide so that the blood remains
- 44. Nitrous oxide can be used as an oxidizer in a rocket motor In vehicle racing, nitrous
- 45. The gas is approved for use as a food additive (also known as E942), specifically as
- 46. Of the entire anthropogenic N2O emission (5.7 teragrams N2O-N per year), agricultural soils provide 3.5 teragrams
- 47. Cumulative effect Recent experiments show that interaction between water vapor, N2O and cosmic radiation increases cloud
- 48. - Other Effects on Climate Tropospheric Ozone Anthropogenic emissions have lead to increase Increases are heterogeneous,
- 49. - Other Effects on Climate Aerosol Effects – Light Scattering Aerosol As was discussed previously in
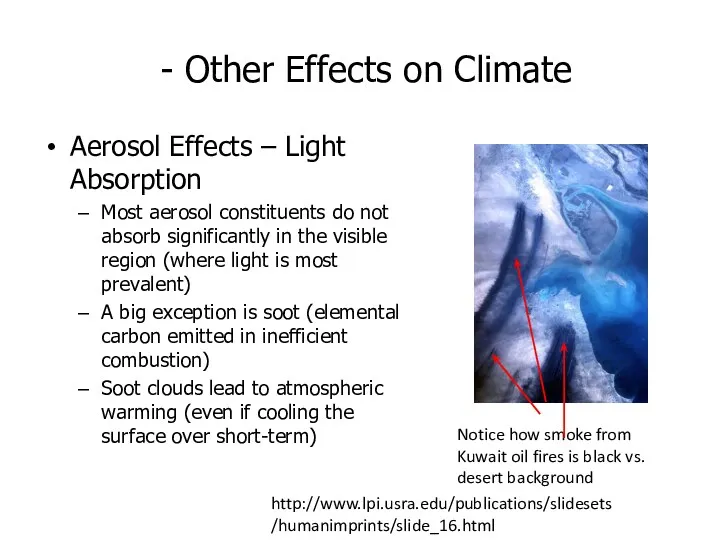
- 50. - Other Effects on Climate Aerosol Effects – Light Absorption Most aerosol constituents do not absorb
- 51. - Other Effects on Climate Indirect Effect of Aerosols One type is through modification of cloud
- 52. Climate Change - Other Effects on Climate Indirect Effect of Aerosols Larger droplets reflect light more
- 53. Aerosol and soot pollutants Can enhance or counteract projected global warming Sulfate particles reflect sunlight Soot
- 55. Feedback Effect The climate system is very complicated. A change in one component of the system
- 56. An example of positive feedback When the climate becomes warmer (either due to the increase of
- 58. Скачать презентацию







































 Азотсодержащие органические соединения. Аминокислоты (часть 2)
Азотсодержащие органические соединения. Аминокислоты (часть 2)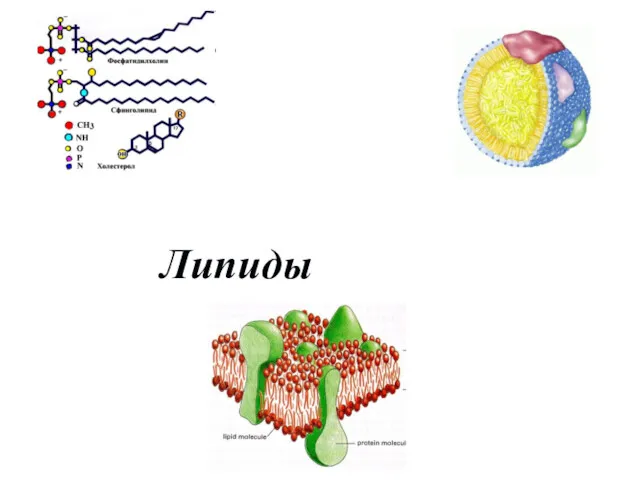 Липиды. Классификация липидов
Липиды. Классификация липидов Электронные конфигурации атомов химических элементов. Графическое изображение электронных конфигураций атомов
Электронные конфигурации атомов химических элементов. Графическое изображение электронных конфигураций атомов Кристаллическое строение и кристаллизация металлов
Кристаллическое строение и кристаллизация металлов Аурум
Аурум Естери. Класифікація та номенклатура естерів
Естери. Класифікація та номенклатура естерів Сравнение нормативов некоторых веществ
Сравнение нормативов некоторых веществ Строение атома
Строение атома Методы разделения белковых смесей. Электрофорез
Методы разделения белковых смесей. Электрофорез Кислоты, их состав и названия
Кислоты, их состав и названия Ненасыщенные (непредельные) алифатические углеводороды. Алкены
Ненасыщенные (непредельные) алифатические углеводороды. Алкены Строение атома. Периодический закон Д. И. Менделеева
Строение атома. Периодический закон Д. И. Менделеева Химическая связь и ее типы. Кристаллические решетки
Химическая связь и ее типы. Кристаллические решетки Теория электролитической диссоциации. Кислотно-основные равновесия в водных растворах
Теория электролитической диссоциации. Кислотно-основные равновесия в водных растворах Металлы в природе. Общие способы их получения
Металлы в природе. Общие способы их получения Фенол и его свойства
Фенол и его свойства Проект на тему Екзо- та ендотермічні реакції на службі людства
Проект на тему Екзо- та ендотермічні реакції на службі людства Частицы вещества: молекула, атом, ион
Частицы вещества: молекула, атом, ион Оксид серы (IV). Сернистая кислота и её соли
Оксид серы (IV). Сернистая кислота и её соли Физические и химические явления. Признаки химических реакций
Физические и химические явления. Признаки химических реакций Некоторые закономерности протекания химических реакций: тепловой эффект реакции, скорость реакции, химическое равновесие
Некоторые закономерности протекания химических реакций: тепловой эффект реакции, скорость реакции, химическое равновесие Склад та властивості основних класів неорганічних сполук
Склад та властивості основних класів неорганічних сполук Хроматографические методы в аналитической химии
Хроматографические методы в аналитической химии Мінеральні добрива
Мінеральні добрива Электролиз. 11 класс
Электролиз. 11 класс Щелочные металлы
Щелочные металлы Периодическая система элементов, предсказание химических свойств элементов на основе таблицы
Периодическая система элементов, предсказание химических свойств элементов на основе таблицы Углекислый газ
Углекислый газ