Содержание
- 2. PH 0101 UNIT 4 LECTURE 7 CRYSTAL DEFECTS AND IMPERFECTIONS An ideal crystal is a perfect
- 3. PH 0101 UNIT 4 LECTURE 7 CRYSTAL DEFECTS AND IMPERFECTIONS The study of imperfections has a
- 4. PH 0101 UNIT 4 LECTURE 7 CRYSTAL DEFECTS AND IMPERFECTIONS Crystal imperfections can be classified on
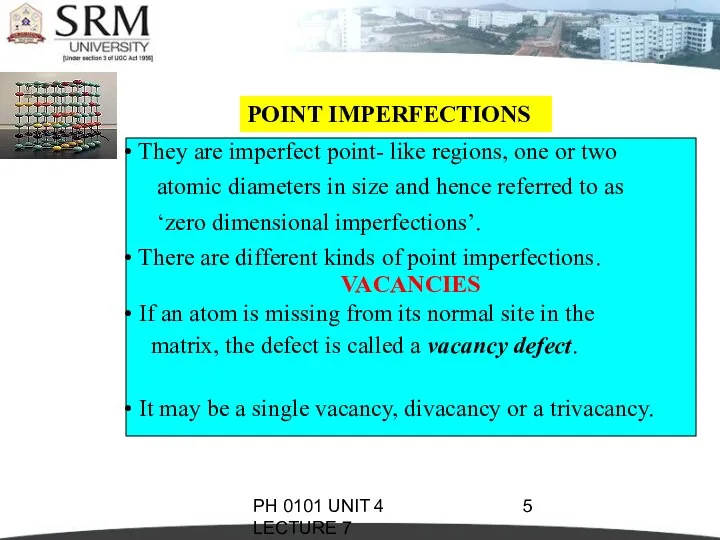
- 5. PH 0101 UNIT 4 LECTURE 7 POINT IMPERFECTIONS They are imperfect point- like regions, one or
- 6. PH 0101 UNIT 4 LECTURE 7 POINT DEFECT-VACANCY
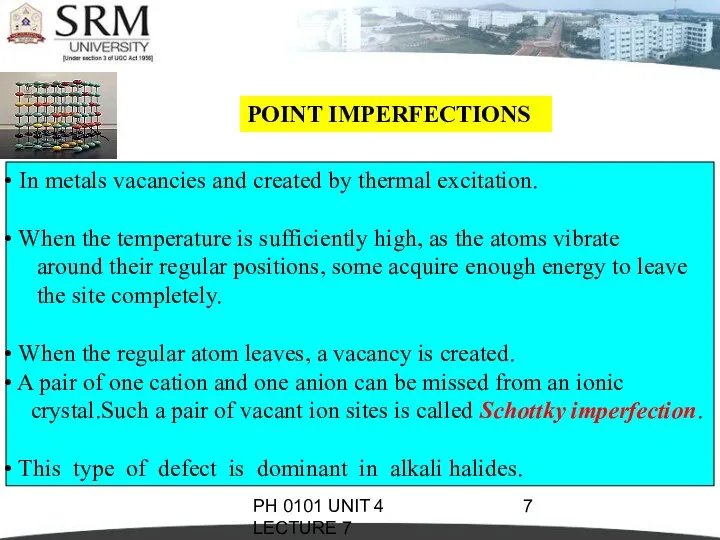
- 7. PH 0101 UNIT 4 LECTURE 7 POINT IMPERFECTIONS In metals vacancies and created by thermal excitation.
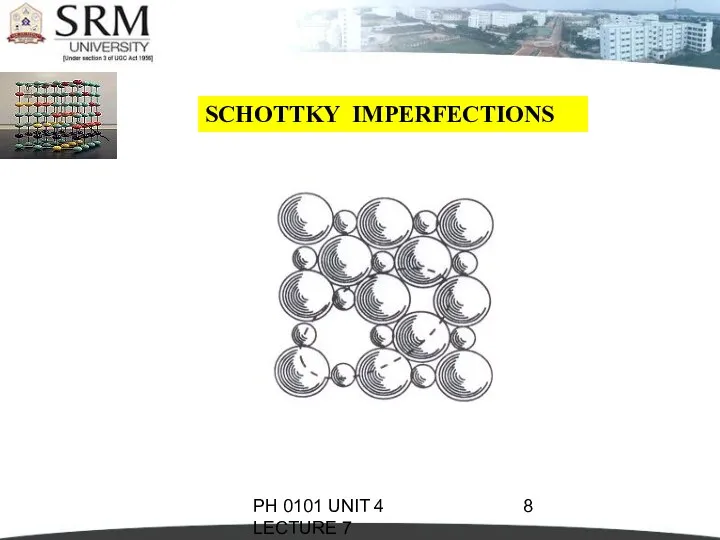
- 8. PH 0101 UNIT 4 LECTURE 7 SCHOTTKY IMPERFECTIONS
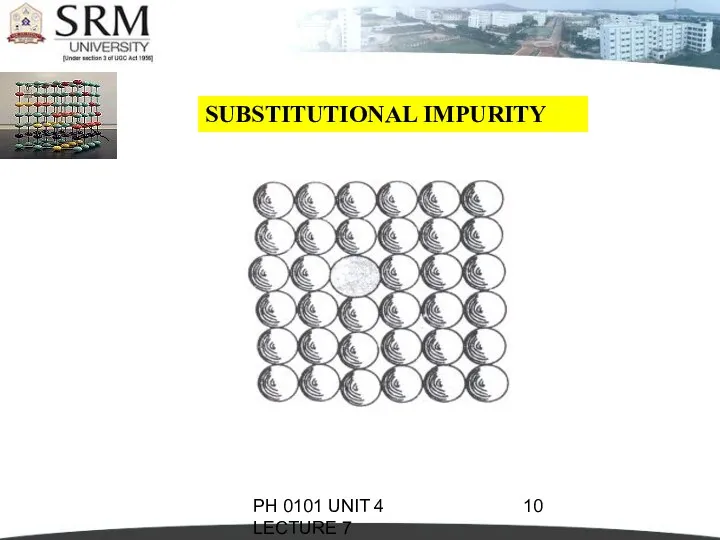
- 9. PH 0101 UNIT 4 LECTURE 7 SUBSTITUTIONAL IMPURITY It refers to a foreign atom that substitutes
- 10. PH 0101 UNIT 4 LECTURE 7 SUBSTITUTIONAL IMPURITY
- 11. PH 0101 UNIT 4 LECTURE 7 INTERSTITIAL IMPURITY An interstitial defect arises when an atom occupies
- 12. PH 0101 UNIT 4 LECTURE 7 INTERSTITIAL IMPURITY
- 13. PH 0101 UNIT 4 LECTURE 7 INTERSTITIAL IMPURITY In ionic crystals, an ion displaced from a
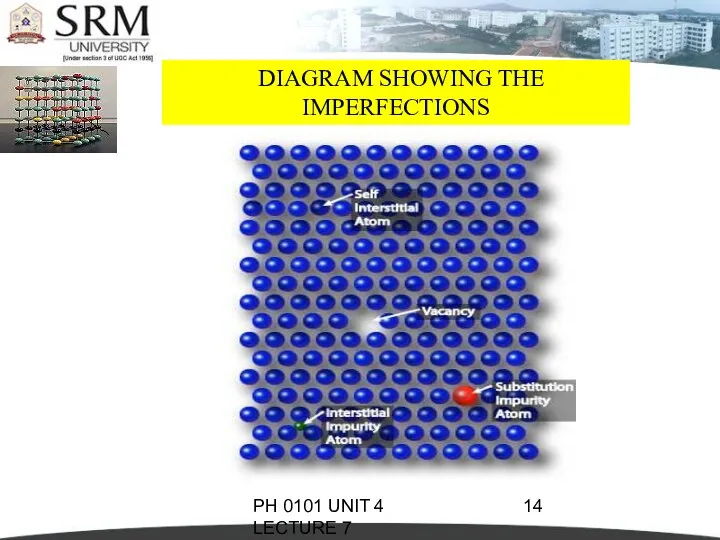
- 14. PH 0101 UNIT 4 LECTURE 7 DIAGRAM SHOWING THE IMPERFECTIONS
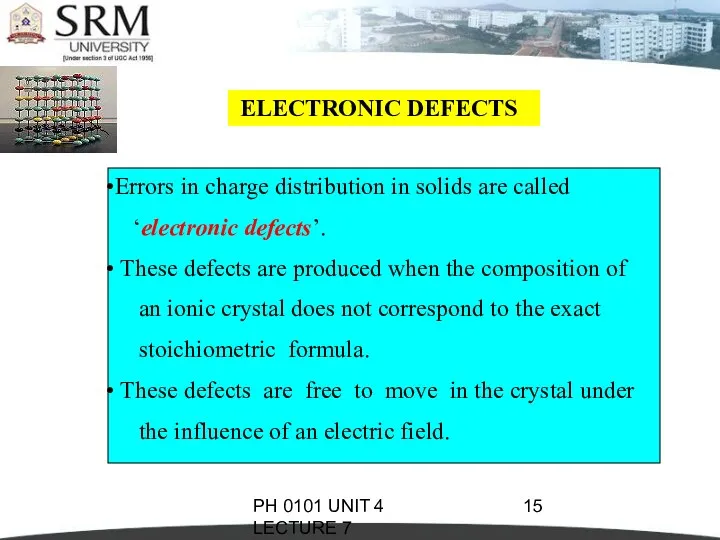
- 15. PH 0101 UNIT 4 LECTURE 7 ELECTRONIC DEFECTS Errors in charge distribution in solids are called
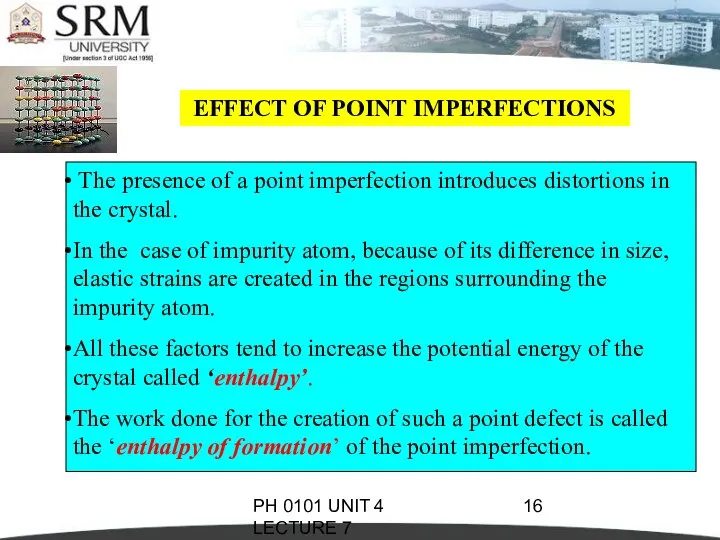
- 16. PH 0101 UNIT 4 LECTURE 7 EFFECT OF POINT IMPERFECTIONS The presence of a point imperfection
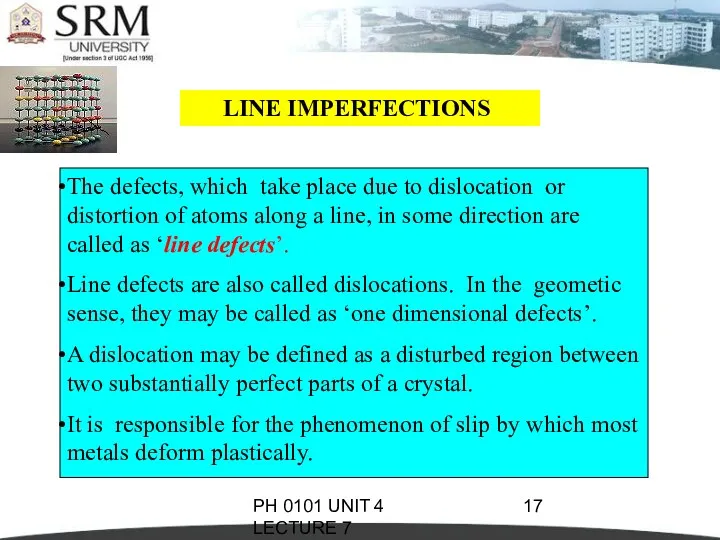
- 17. PH 0101 UNIT 4 LECTURE 7 LINE IMPERFECTIONS The defects, which take place due to dislocation
- 18. PH 0101 UNIT 4 LECTURE 7 LINE IMPERFECTIONS The two types of dislocations are, Edge dislocation
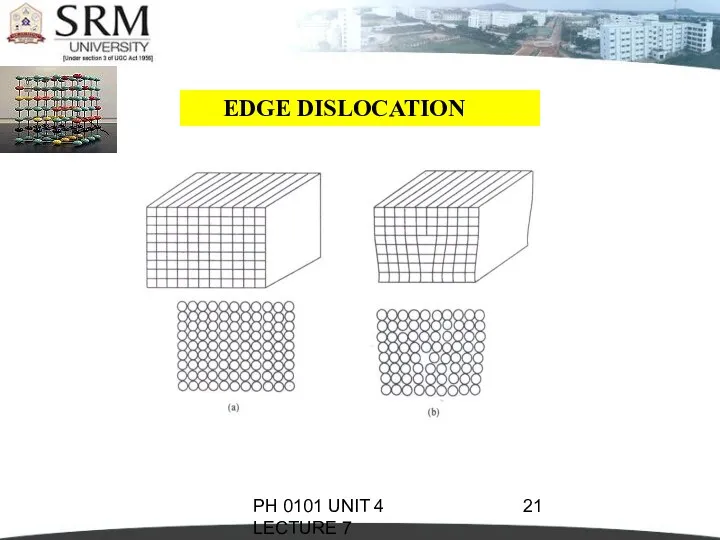
- 19. PH 0101 UNIT 4 LECTURE 7 EDGE DISLOCATION In perfect crystal, atoms are arranged in both
- 20. PH 0101 UNIT 4 LECTURE 7 The distorted configuration extends all along the edge into the
- 21. PH 0101 UNIT 4 LECTURE 7 EDGE DISLOCATION
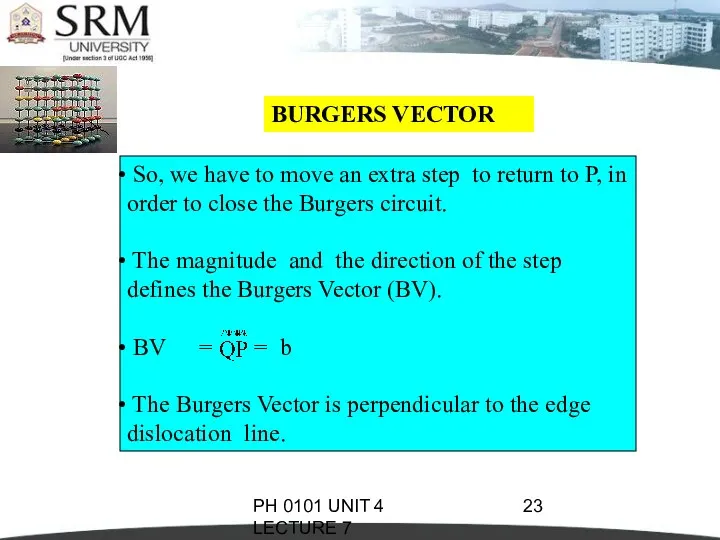
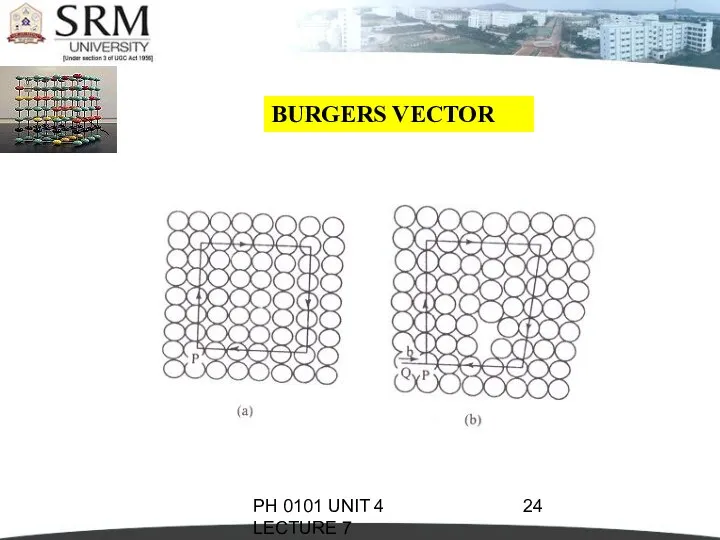
- 22. PH 0101 UNIT 4 LECTURE 7 BURGERS VECTOR The magnitude and the direction of the displacement
- 23. PH 0101 UNIT 4 LECTURE 7 BURGERS VECTOR So, we have to move an extra step
- 24. PH 0101 UNIT 4 LECTURE 7 BURGERS VECTOR
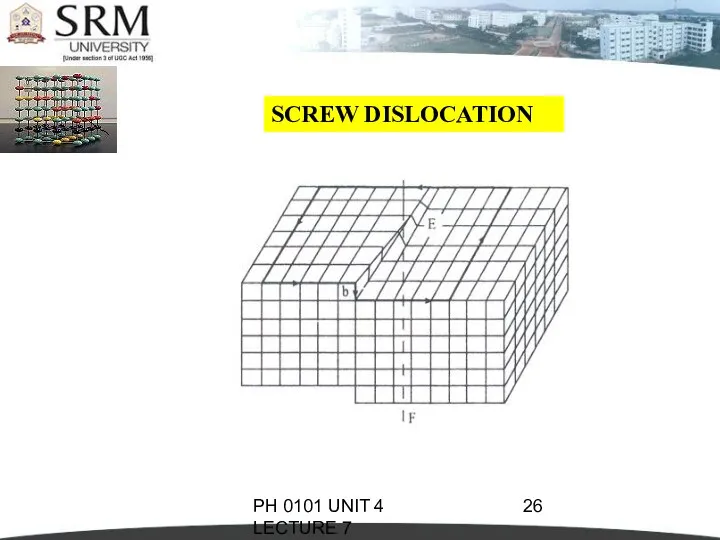
- 25. PH 0101 UNIT 4 LECTURE 7 SCREW DISLOCATION In this dislocation, the atoms are displaced in
- 26. PH 0101 UNIT 4 LECTURE 7 SCREW DISLOCATION
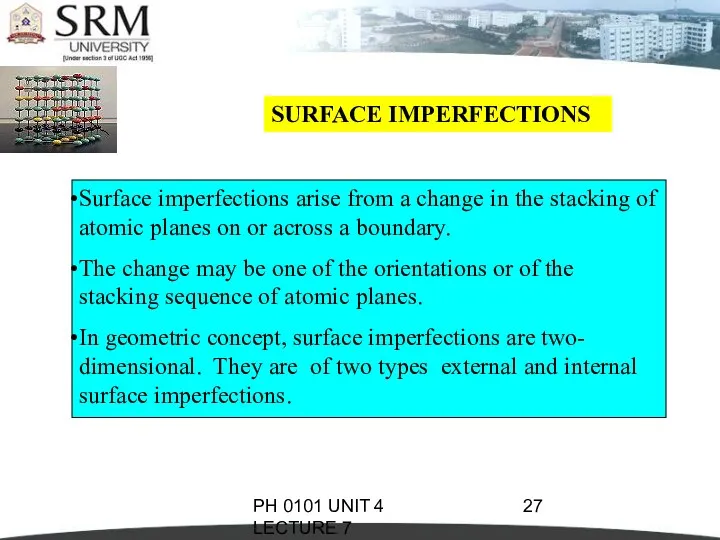
- 27. PH 0101 UNIT 4 LECTURE 7 SURFACE IMPERFECTIONS Surface imperfections arise from a change in the
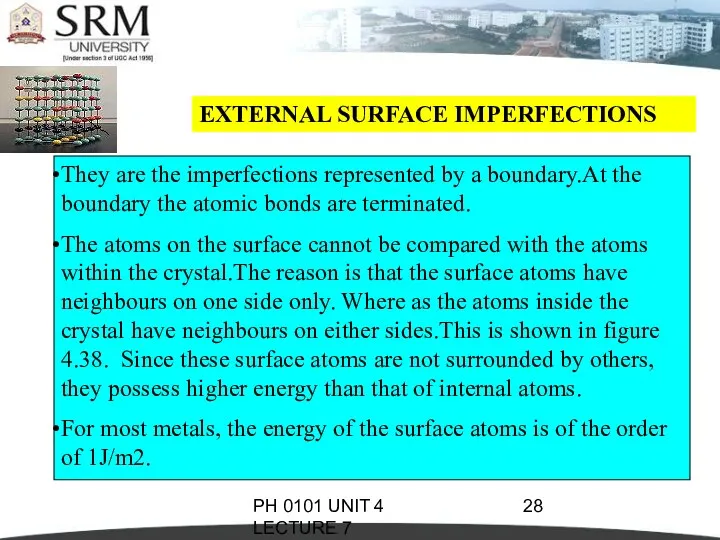
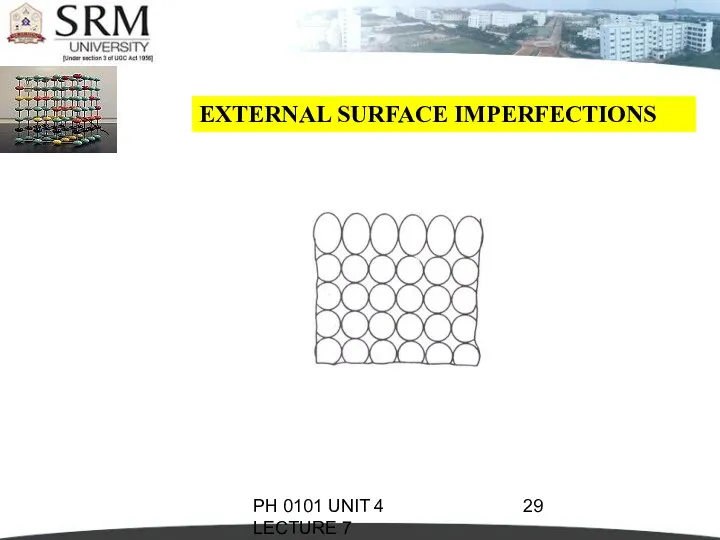
- 28. PH 0101 UNIT 4 LECTURE 7 EXTERNAL SURFACE IMPERFECTIONS They are the imperfections represented by a
- 29. PH 0101 UNIT 4 LECTURE 7 EXTERNAL SURFACE IMPERFECTIONS
- 30. PH 0101 UNIT 4 LECTURE 7 INTERNAL SURFACE IMPERFECTIONS Internal surface imperfections are the imperfections which
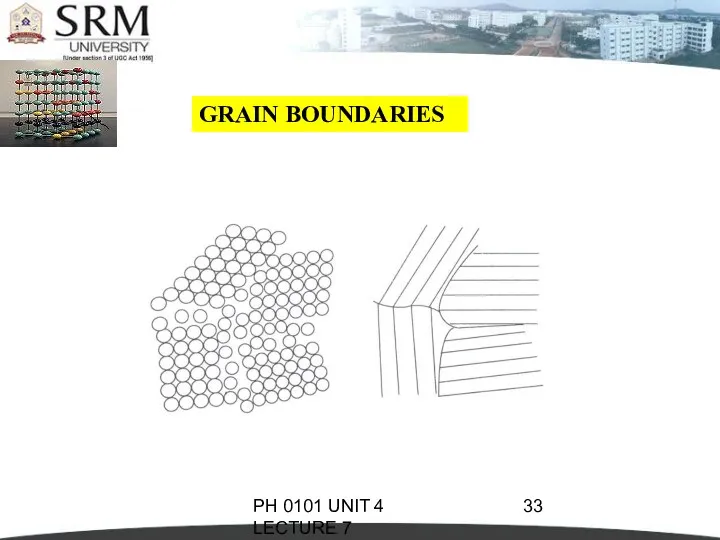
- 31. PH 0101 UNIT 4 LECTURE 7 GRAIN BOUNDARIES They are the imperfections which separate crystals or
- 32. PH 0101 UNIT 4 LECTURE 7 GRAIN BOUNDARIES These positions at the boundary region between two
- 33. PH 0101 UNIT 4 LECTURE 7 GRAIN BOUNDARIES
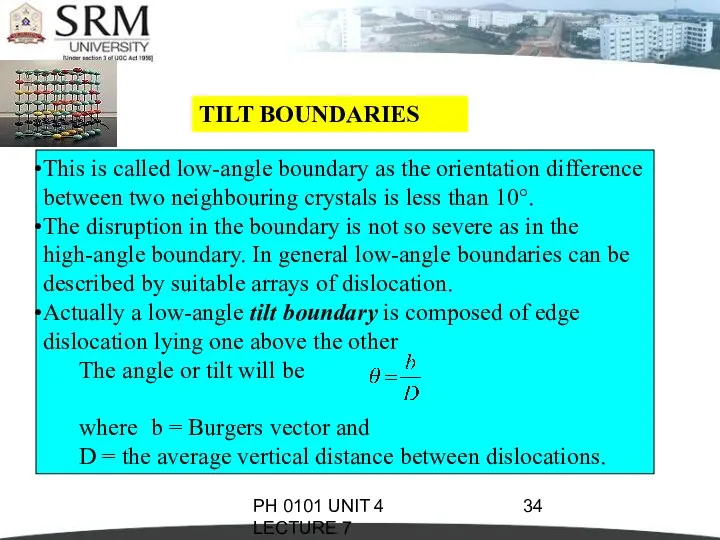
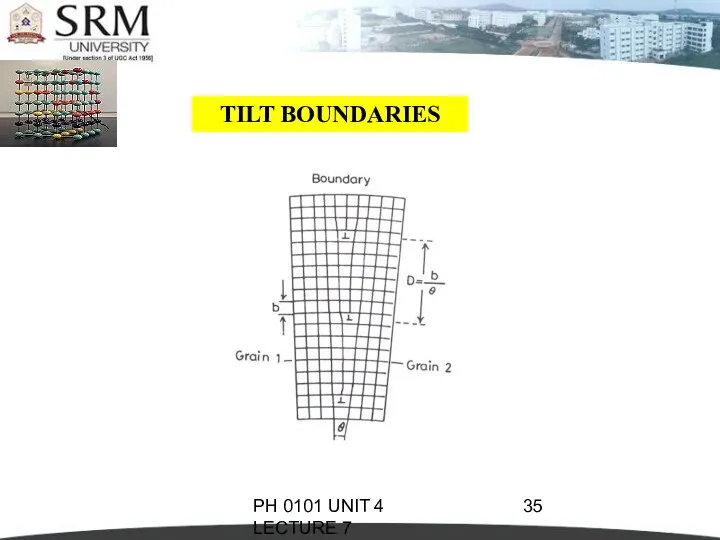
- 34. PH 0101 UNIT 4 LECTURE 7 TILT BOUNDARIES This is called low-angle boundary as the orientation
- 35. PH 0101 UNIT 4 LECTURE 7 TILT BOUNDARIES
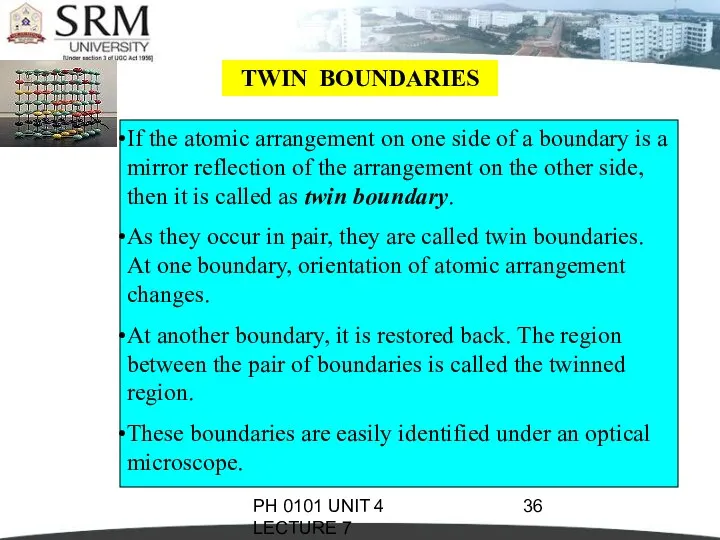
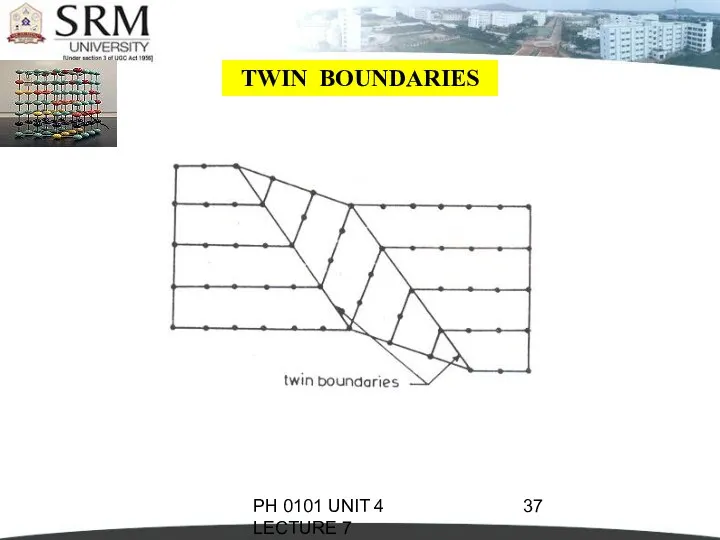
- 36. PH 0101 UNIT 4 LECTURE 7 TWIN BOUNDARIES If the atomic arrangement on one side of
- 37. PH 0101 UNIT 4 LECTURE 7 TWIN BOUNDARIES
- 38. PH 0101 UNIT 4 LECTURE 7 STACKING FAULTS Whenever the stacking of atomic planes is not
- 39. PH 0101 UNIT 4 LECTURE 7 STACKING FAULTS
- 40. PH 0101 UNIT 4 LECTURE 7 VOLUME IMPERFECTIONS Volume defects such as cracks may arise in
- 42. Скачать презентацию














 Химический элемент таблицы Менделеева - Азот
Химический элемент таблицы Менделеева - Азот Гетерофункциональные соединения, участвующие в процессах жизнедеятельности
Гетерофункциональные соединения, участвующие в процессах жизнедеятельности Строение атома азота
Строение атома азота Классификация химических реакций
Классификация химических реакций Синтетические топлива
Синтетические топлива Бейорганикалық заттар технологиясындағы жүйелерді термодинамикалық талдау
Бейорганикалық заттар технологиясындағы жүйелерді термодинамикалық талдау Physiology lab
Physiology lab Металлы в нашей жизни
Металлы в нашей жизни Классификация химических реакций
Классификация химических реакций Фенолы. Классификация фенолов
Фенолы. Классификация фенолов ЭЛЕКТРОЛИЗ
ЭЛЕКТРОЛИЗ Химическая термодинамика. Термохимия. Лекция 6
Химическая термодинамика. Термохимия. Лекция 6 Аналитические методы
Аналитические методы Физические и химические свойства алкенов
Физические и химические свойства алкенов Комплесные соединения
Комплесные соединения Химическая кинетика. Закон действующих масс для скорости реакции
Химическая кинетика. Закон действующих масс для скорости реакции Химическая технология. Введение
Химическая технология. Введение Генетические ряды металлов, образующих нерастворимый гидроксид
Генетические ряды металлов, образующих нерастворимый гидроксид Химические свойства основных неорганических соединений в свете ЭД и ОВР
Химические свойства основных неорганических соединений в свете ЭД и ОВР Железо, его характеристики, свойства и соединения
Железо, его характеристики, свойства и соединения Электронные конфигурации атомов. Периодический Закон. Периодическая система Д.И. Менделеева. Химическая связь
Электронные конфигурации атомов. Периодический Закон. Периодическая система Д.И. Менделеева. Химическая связь Chemical reaction rate. Influence of conditions on the rate of chemical reactions. Catalysis. Topic 3.2
Chemical reaction rate. Influence of conditions on the rate of chemical reactions. Catalysis. Topic 3.2 Относительная атомная и относительная молекулярная масса
Относительная атомная и относительная молекулярная масса ПЛАСТИК НОВЫЙ
ПЛАСТИК НОВЫЙ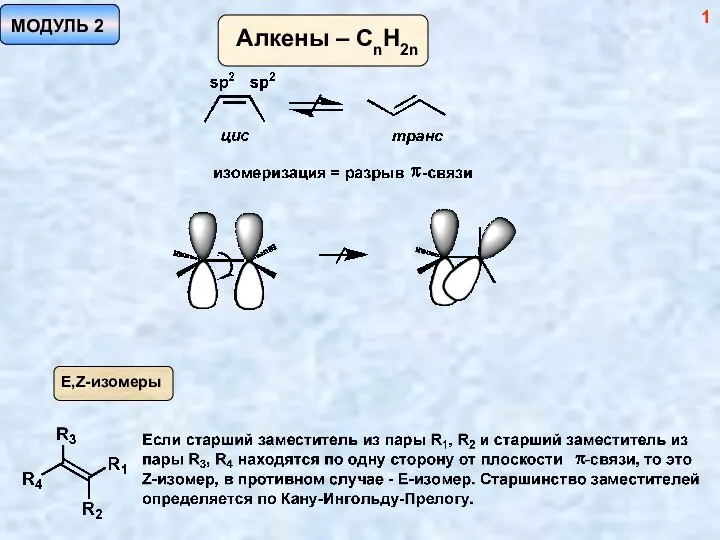 Методы синтеза алкенов. (Модуль 2)
Методы синтеза алкенов. (Модуль 2)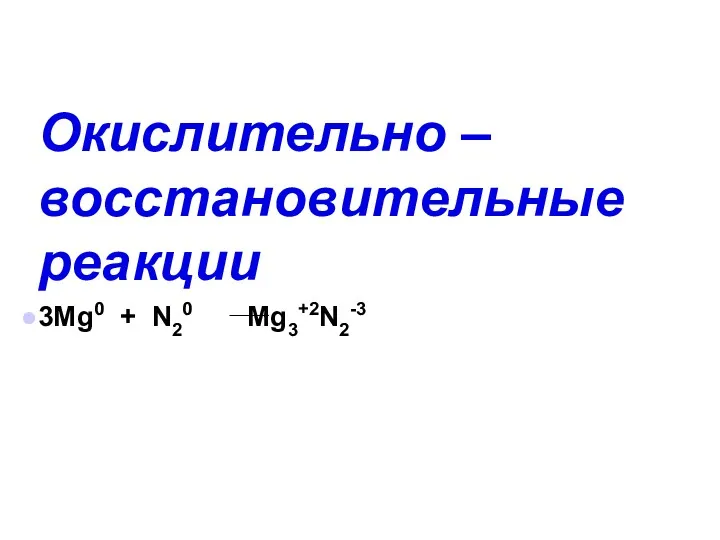 Окислительно – восстановительные реакции
Окислительно – восстановительные реакции Базовое нефтехимическое сырье
Базовое нефтехимическое сырье Химические уравнения
Химические уравнения