Содержание
- 2. INTRODUCTION Nanotechnology was born as a science very recently. It is one of the greatest discoveries
- 3. INTRODUCTION Advances for the industry: Materials with new properties not developed until now. Stronger materials than
- 4. INTRODUCTION Richard Feynman was the first to refer the possibilities of nanoscience when in 1959 he
- 5. OBJECTIVES To explain formation and development of Nanochemistry. To give an overview about Nanoparticle as a
- 6. OUTLINE Introduction. Formation and development of Nanochemistry. Nanoparticle as a structural unit of new substances. Classification
- 7. Formation and development of Nanochemistry Nanoscience: study of phenomena and manipulation of materials at atomic, molecular
- 8. Formation and development of Nanochemistry Nanoparticles can be defined as particles with at least one of
- 9. Classification of nanoparticles The US Environmental Agency: Carbon based materials: with spherical, ellipsoidal or tubular forms.
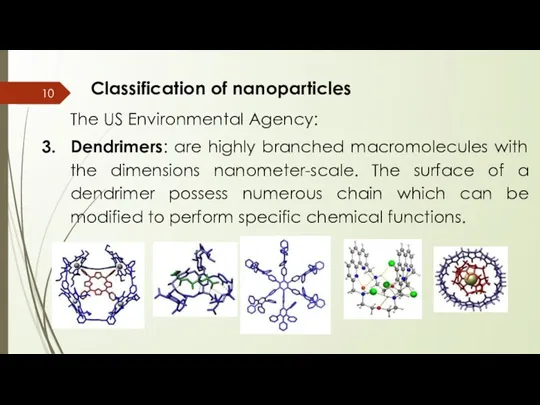
- 10. Classification of nanoparticles The US Environmental Agency: Dendrimers: are highly branched macromolecules with the dimensions nanometer-scale.
- 11. Classification of nanoparticles The US Environmental Agency: 4. Composites: Nanocomposite can be described as a multiphase
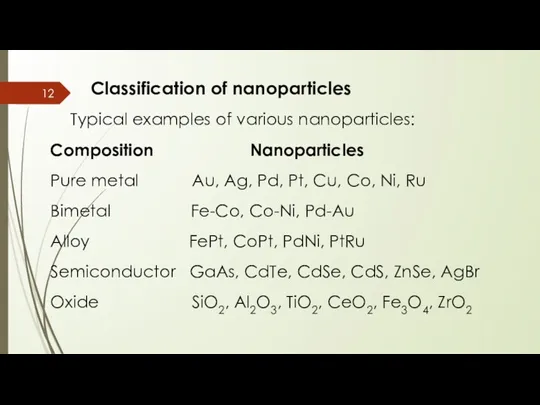
- 12. Classification of nanoparticles Typical examples of various nanoparticles: Composition Nanoparticles Pure metal Au, Ag, Pd, Pt,
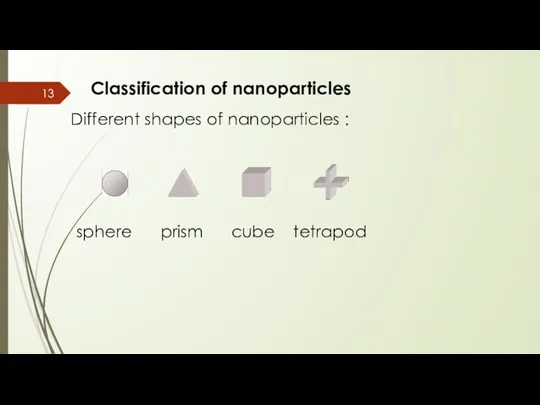
- 13. Classification of nanoparticles Different shapes of nanoparticles : sphere prism cube tetrapod
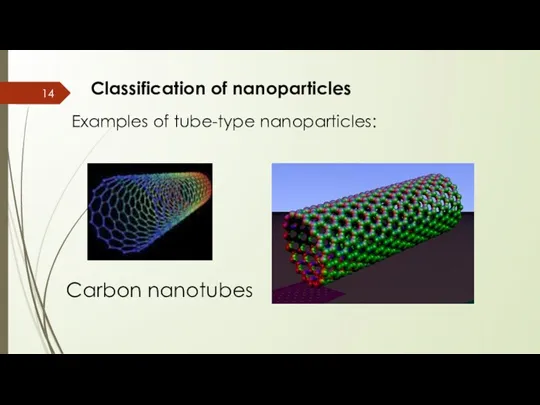
- 14. Classification of nanoparticles Examples of tube-type nanoparticles: Carbon nanotubes
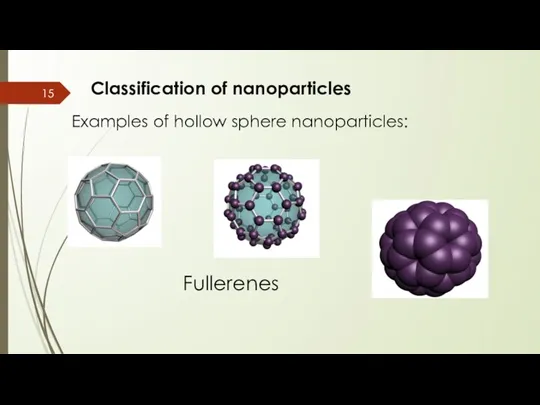
- 15. Classification of nanoparticles Examples of hollow sphere nanoparticles: Fullerenes
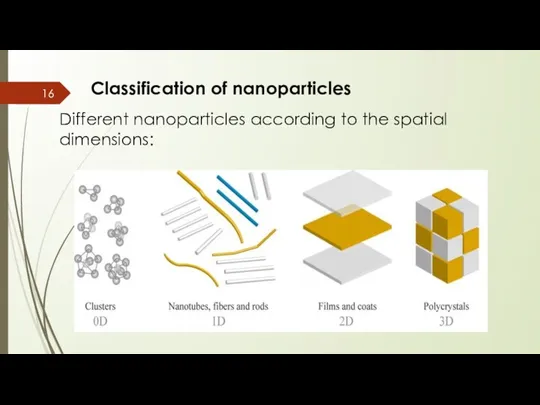
- 16. Classification of nanoparticles Different nanoparticles according to the spatial dimensions:
- 17. Classification of nanoparticles Different nanoparticles according to the spatial dimensions: Zero dimensional(0-D): These nanomaterials have Nano-dimensions
- 18. Classification of nanoparticles Different nanoparticles according to the spatial dimensions: Two dimensional(2-D): In this type of
- 19. Properties of nanoparticles The causes of these behavioral differences in their properties are mainly two: The
- 20. Properties of nanoparticles Variation of the surface area 13 atoms (12 on the surface) 92% 55
- 21. Properties of nanoparticles Physical properties: The melting point Electrical and thermal conductivity Chemical properties Mechanical properties:
- 22. Conclusions A nanomaterial differs from a conventional polycrystalline material not only because of the size of
- 24. Скачать презентацию












 Физико-химические свойства жиров
Физико-химические свойства жиров Обчислення за хімічними рівняннями відносного виходу продукту реакції
Обчислення за хімічними рівняннями відносного виходу продукту реакції Соли, их классификация и свойства
Соли, их классификация и свойства Халькогены. Кислород
Халькогены. Кислород Алкадиены
Алкадиены Химические свойства солей
Химические свойства солей Общая характеристика элементов VA -группы. Азот, распространение, физические и химические свойства. Круговорот в природе
Общая характеристика элементов VA -группы. Азот, распространение, физические и химические свойства. Круговорот в природе Полимеры. Структура и свойства
Полимеры. Структура и свойства Амины
Амины Химическая очистка воды
Химическая очистка воды Кристаллохимия негіздері
Кристаллохимия негіздері Этиловый спирт в жизни человека
Этиловый спирт в жизни человека 20231104_prezentatsiya_teoriya_elektroliticheskoy_dissotsiatsii
20231104_prezentatsiya_teoriya_elektroliticheskoy_dissotsiatsii Crystal structure and surface phase composition of palladium oxides thin films for gas sensors
Crystal structure and surface phase composition of palladium oxides thin films for gas sensors Природные источники углеводородов
Природные источники углеводородов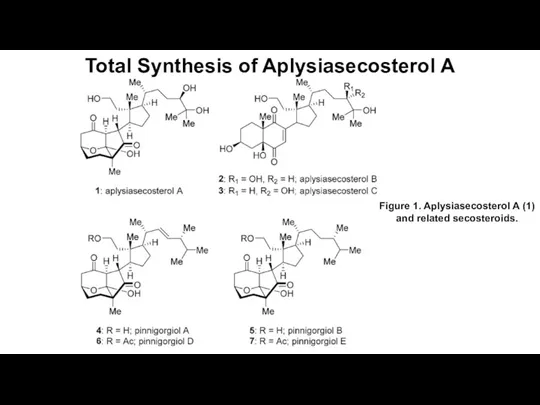 Total Synthesis of Aplysiasecosterol A
Total Synthesis of Aplysiasecosterol A Классификация неорганический веществ
Классификация неорганический веществ Изотопы химических элементов
Изотопы химических элементов Окислительно - восстановительные реакции (ОВР) (часть 1)
Окислительно - восстановительные реакции (ОВР) (часть 1) Кислотно-основные (протолитические) равновесия
Кислотно-основные (протолитические) равновесия Каталитикалық риформинг
Каталитикалық риформинг Неметаллические материалы
Неметаллические материалы Кристаллическое состояние вещества
Кристаллическое состояние вещества Концентрация кобальта
Концентрация кобальта Введение в минералогию. Генезис минералов
Введение в минералогию. Генезис минералов Sm-Nd метод
Sm-Nd метод Кислород, его характеристика, получение и свойства
Кислород, его характеристика, получение и свойства Периодический закон и периодическая система химических элементов
Периодический закон и периодическая система химических элементов