Содержание
- 2. Over 80 million tonnes of poly(ethene), often known as polyethylene and polythene, is manufactured each year
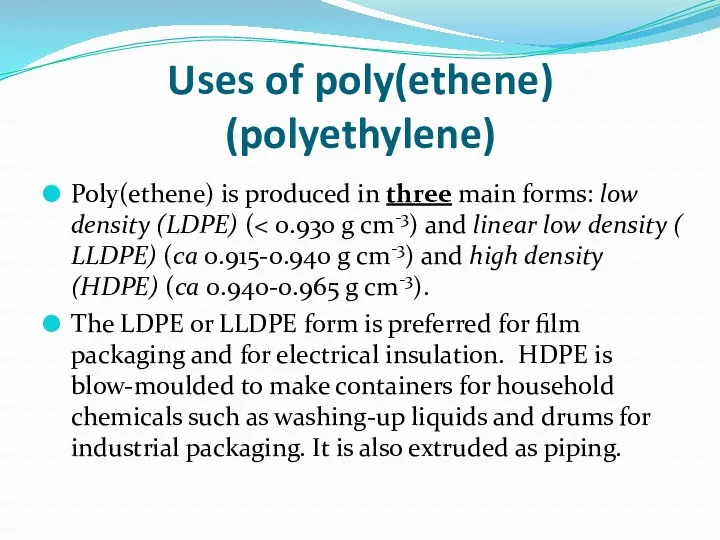
- 3. Uses of poly(ethene) (polyethylene) Poly(ethene) is produced in three main forms: low density (LDPE) ( The
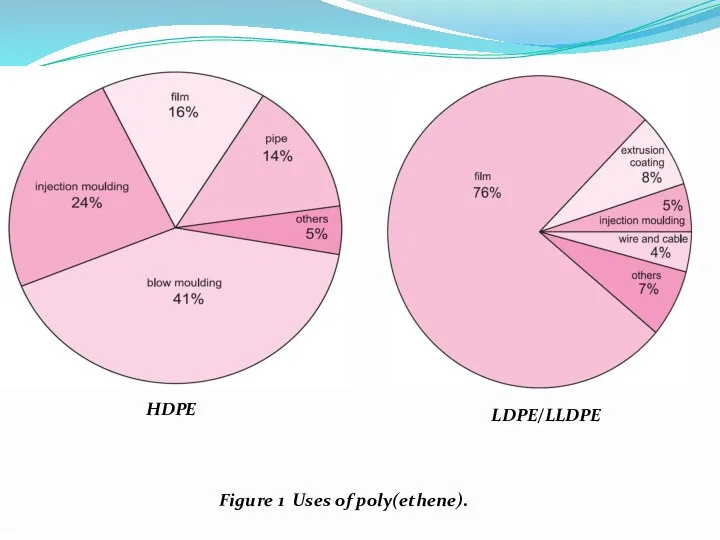
- 4. HDPE LDPE/LLDPE Figure 1 Uses of poly(ethene).
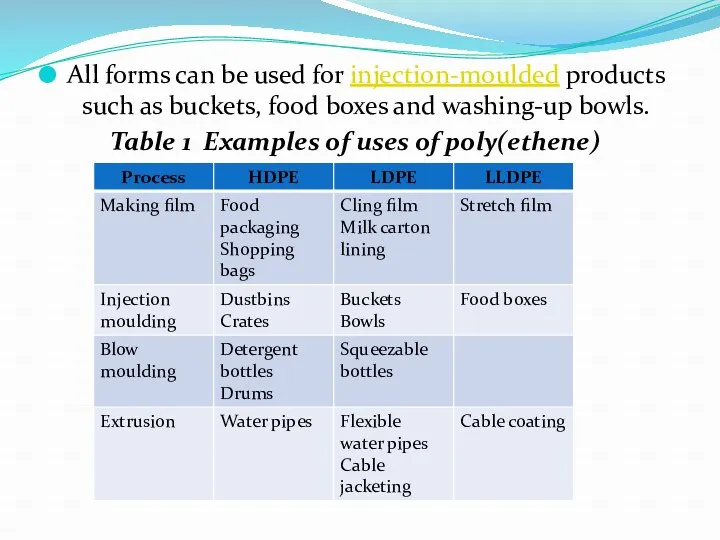
- 5. All forms can be used for injection-moulded products such as buckets, food boxes and washing-up bowls.
- 6. Poly(ethene) is used to make large water pipes and far smaller pipes.
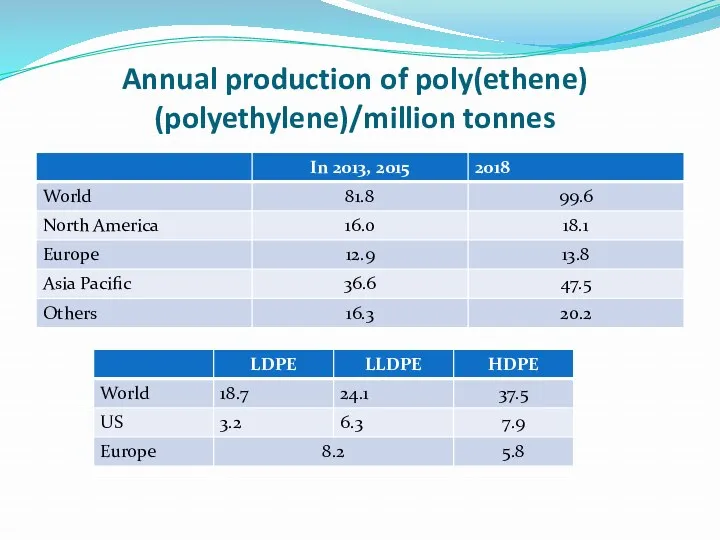
- 7. Annual production of poly(ethene) (polyethylene)/million tonnes
- 8. Many plants can produce both forms of poly(ethene) and alter the amount that they produce of
- 9. Manufacture of poly(ethene) (polyethylene) Poly(ethene) is made by several methods by addition polymerization of ethene, which
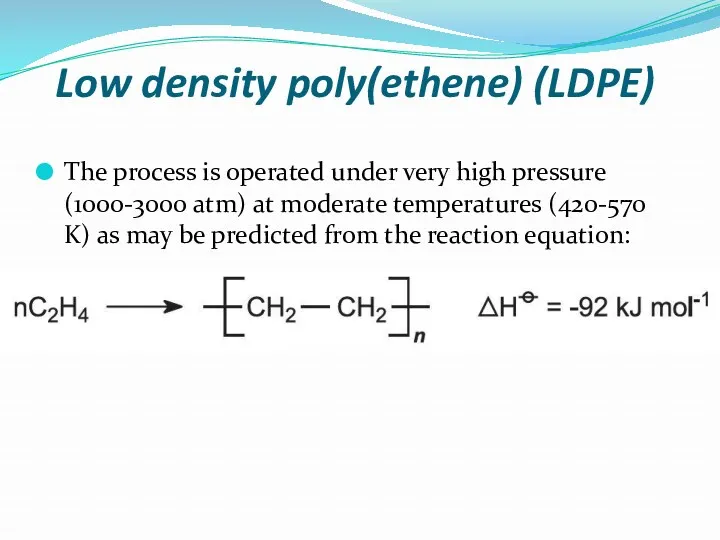
- 10. Low density poly(ethene) (LDPE) The process is operated under very high pressure (1000-3000 atm) at moderate
- 11. This is a radical polymerization process and an initiator, such as a small amount of oxygen,
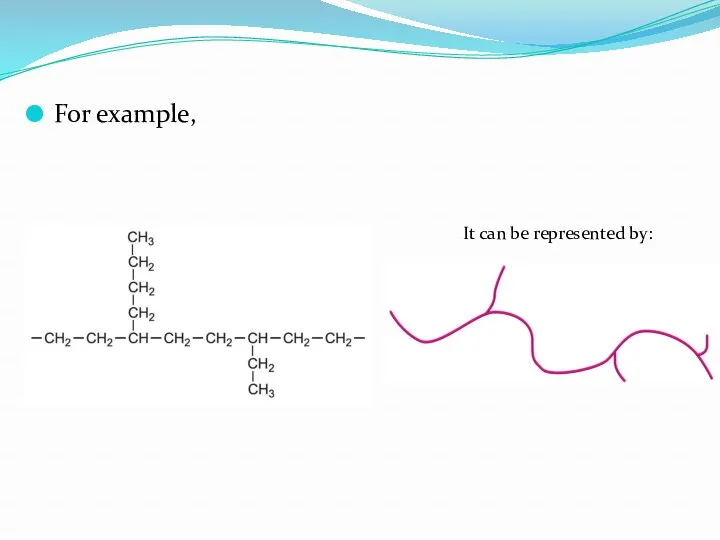
- 12. For example, It can be represented by:
- 13. There are about 20 branches per 1000 carbon atoms. The relative molecular mass, and the branching,
- 14. High density poly(ethene) (HDPE) Two types of catalyst are used principally in the manufacture of HDPE:
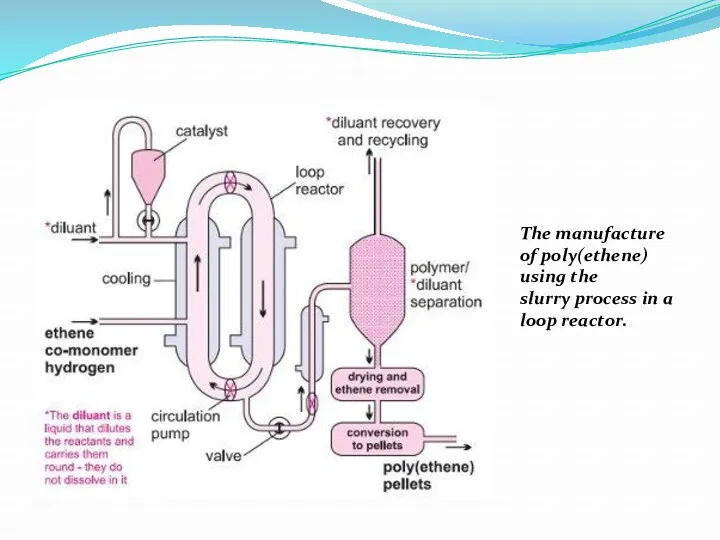
- 15. I. Slurry process (using either CSTR (continuous stirred tank reactor) or a loop) The Ziegler-Natta catalyst,
- 16. The manufacture of poly(ethene) using the slurry process in a loop reactor.
- 17. II. Solution process The second method involves passing ethene and hydrogen under pressure into a solution
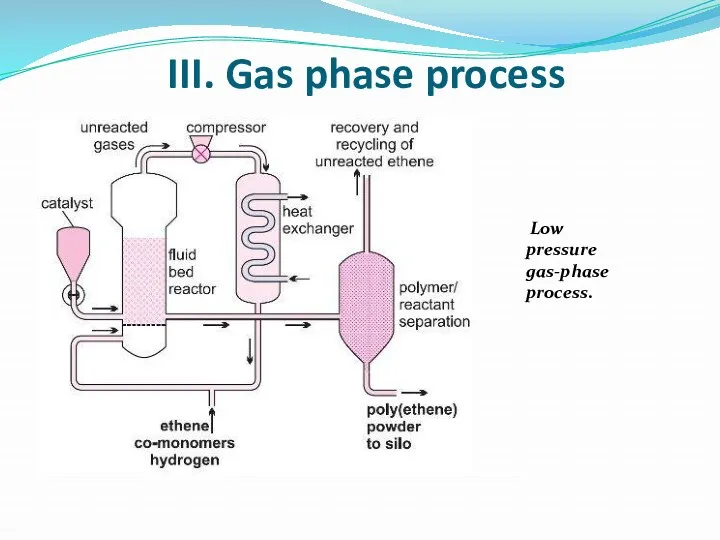
- 18. III. Gas phase process Low pressure gas-phase process.
- 19. A mixture of ethene and hydrogen is passed over a Phillips catalyst in a fixed bed
- 20. Granules of poly(ethene) which are then used to make film, extruded into pipes or moulded.
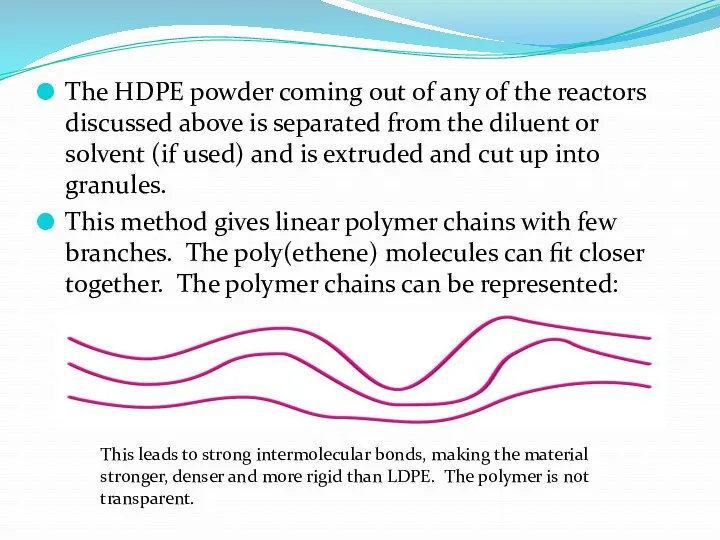
- 21. The HDPE powder coming out of any of the reactors discussed above is separated from the
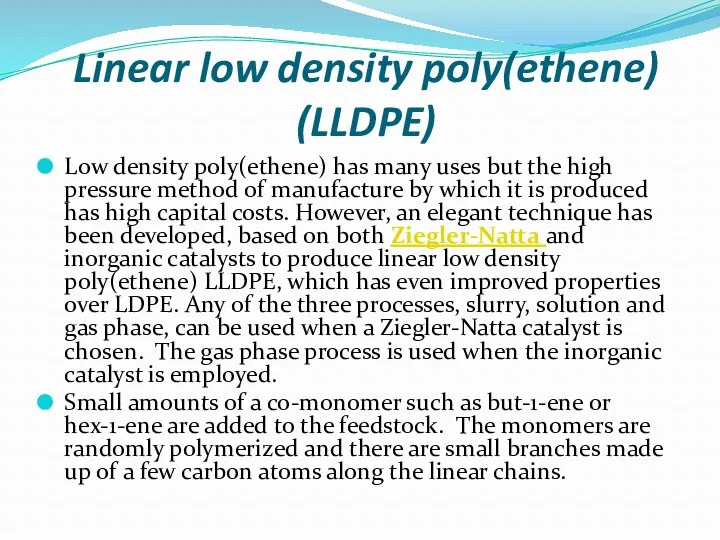
- 22. Linear low density poly(ethene) (LLDPE) Low density poly(ethene) has many uses but the high pressure method
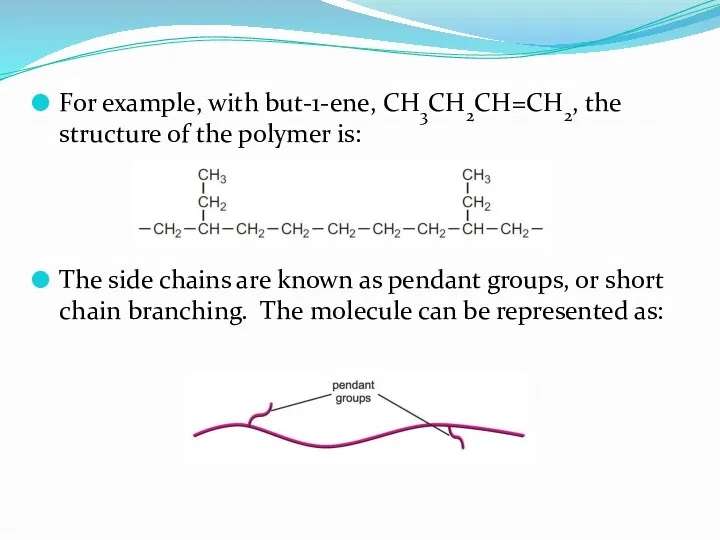
- 23. For example, with but-1-ene, CH3CH2CH=CH2, the structure of the polymer is: The side chains are known
- 24. The structure is essentially linear but because of the short chain branching it has a low
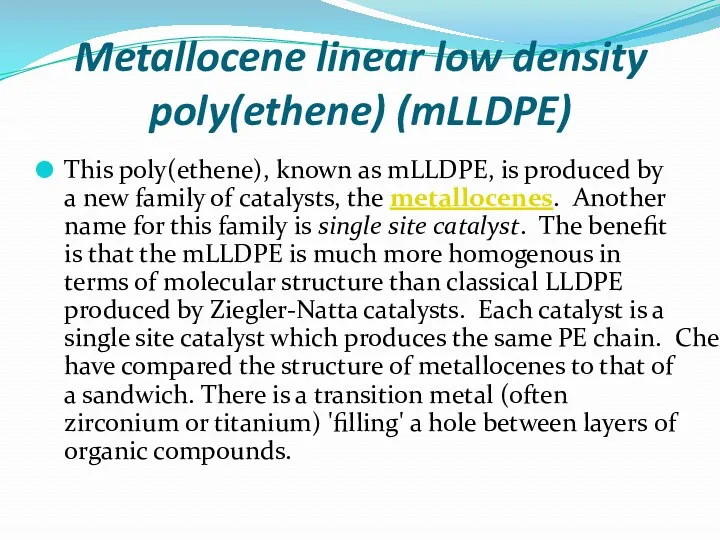
- 25. Metallocene linear low density poly(ethene) (mLLDPE) This poly(ethene), known as mLLDPE, is produced by a new
- 26. The catalysts are even more specific than the original Ziegler-Natta and it is possible to control
- 28. Скачать презентацию








 Гигиена питания школьников
Гигиена питания школьников Растворы ВМС
Растворы ВМС Химические свойства спиртов. Химические свойства предельных одноатомных спиртов
Химические свойства спиртов. Химические свойства предельных одноатомных спиртов Введение в специальность. Химическая технология
Введение в специальность. Химическая технология Важнейшие химические понятия и законы. Атом, химический элемент. Изотопы. Простые и сложные вещества. Основные законы химии
Важнейшие химические понятия и законы. Атом, химический элемент. Изотопы. Простые и сложные вещества. Основные законы химии Дробный метод анализа металлических ядов в минерализате (деструктате) (Продолжение)
Дробный метод анализа металлических ядов в минерализате (деструктате) (Продолжение) Будова електронних оболонок атомів
Будова електронних оболонок атомів Растворы. Приготовление растворов
Растворы. Приготовление растворов Неоднородные системы, их классификация, методы разделения. Лекция 4
Неоднородные системы, их классификация, методы разделения. Лекция 4 Галогены. Строение атомов фтора и хлора
Галогены. Строение атомов фтора и хлора Жиры. Липиды и липоиды
Жиры. Липиды и липоиды Аммиак: состав, строение, свойства, применение
Аммиак: состав, строение, свойства, применение Химическая связь
Химическая связь Школьное мероприятие Д. И. Менделеев в высказываниях
Школьное мероприятие Д. И. Менделеев в высказываниях Поверхностные явления
Поверхностные явления Горные породы
Горные породы Алкадиены, нафтены
Алкадиены, нафтены Алкадиены (диены, диеновые углеводороды)
Алкадиены (диены, диеновые углеводороды) Оксиды. Номенклатура, классификация, физические свойства
Оксиды. Номенклатура, классификация, физические свойства Электрохимические процессы
Электрохимические процессы Получение и приминение этилена
Получение и приминение этилена Строение электронных оболочек атомов
Строение электронных оболочек атомов Электролитическая диссоциация. Занятие 14
Электролитическая диссоциация. Занятие 14 Типы химических реакций
Типы химических реакций Соли-электролиты
Соли-электролиты Основные классы неорганических и органических соединений
Основные классы неорганических и органических соединений Химический элемент хлор
Химический элемент хлор Непредельные углеводороды. Алкены
Непредельные углеводороды. Алкены