Содержание
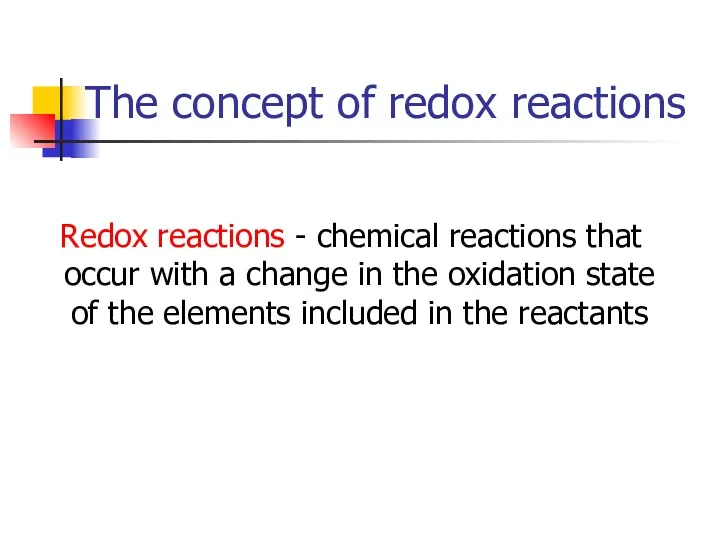
- 2. The concept of redox reactions Redox reactions - chemical reactions that occur with a change in
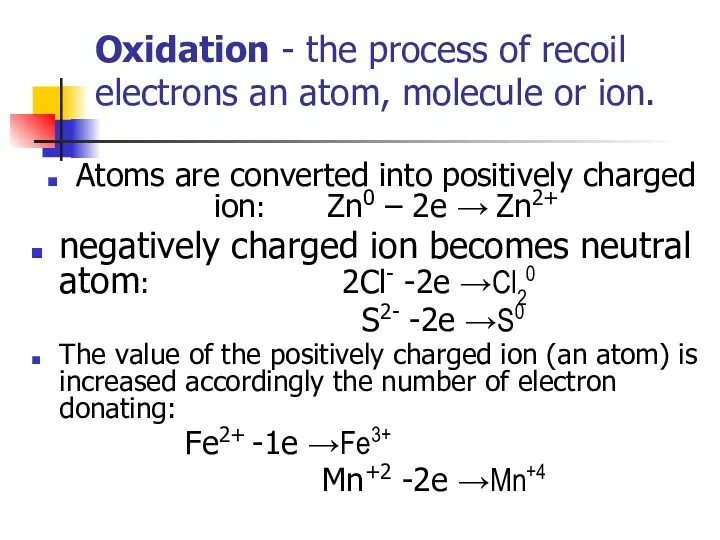
- 3. Oxidation - the process of recoil electrons an atom, molecule or ion. Atoms are converted into
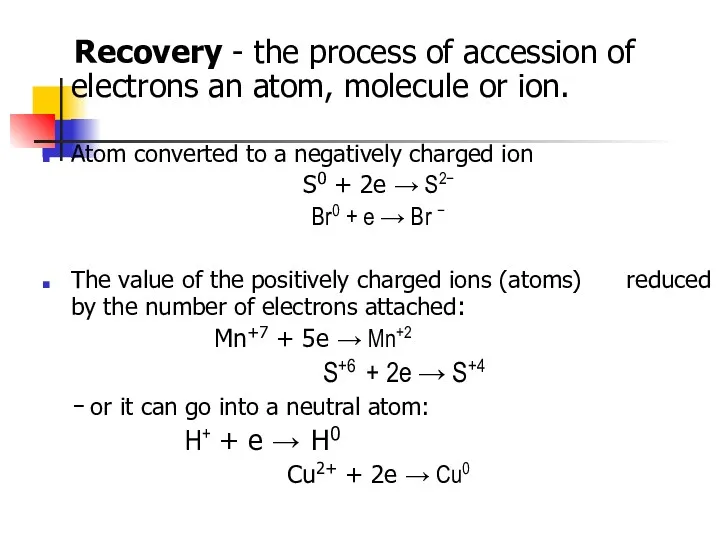
- 4. Recovery - the process of accession of electrons an atom, molecule or ion. Atom converted to
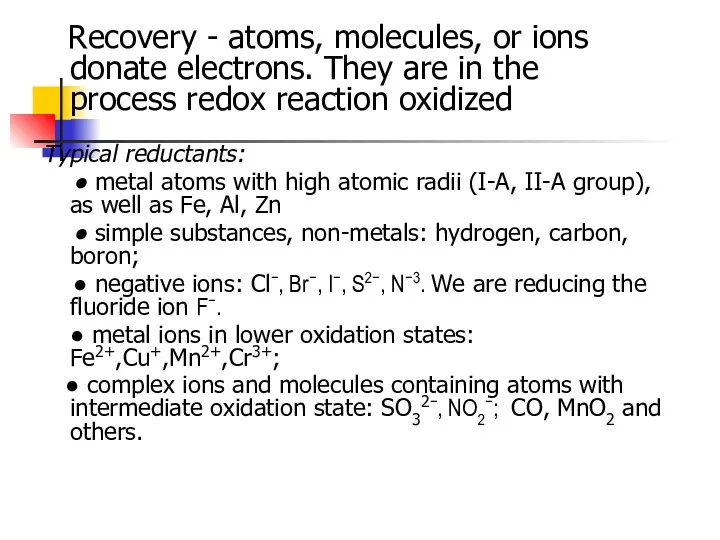
- 5. Recovery - atoms, molecules, or ions donate electrons. They are in the process redox reaction oxidized
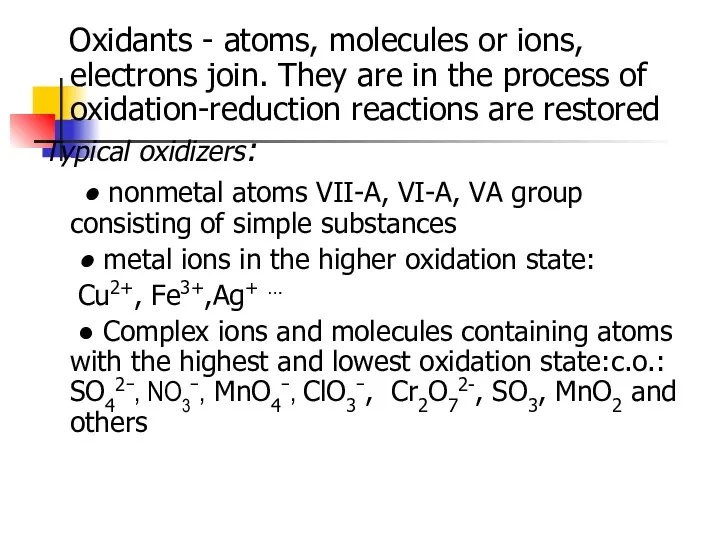
- 6. Oxidants - atoms, molecules or ions, electrons join. They are in the process of oxidation-reduction reactions
- 7. On the display of the redox properties of the effect of such factors as the stability
- 8. The degree of oxidation of sulfur: -2,0,+4,+6 Н2S-2 - reductant 2Н2S+3O2=2H2O+2SO2 S0,S+4O2 – oxidant and reductant
- 9. Определение степеней окисления атомов химических элементов The oxidation state of atoms of chemical elements in the
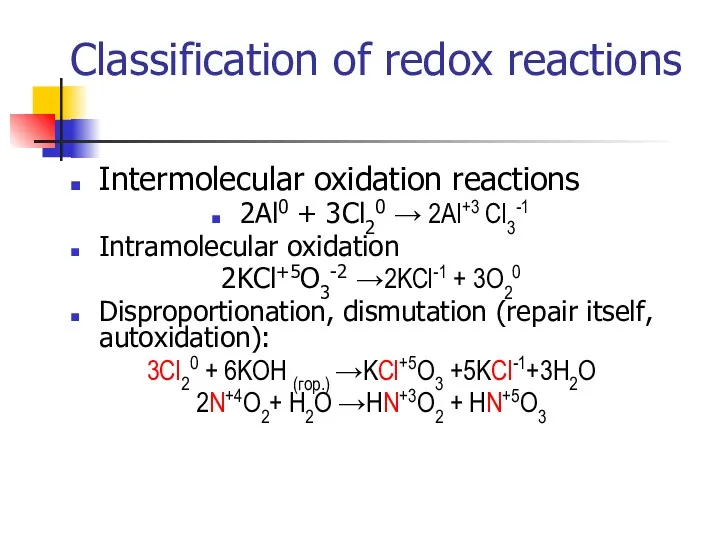
- 10. Classification of redox reactions Intermolecular oxidation reactions 2Al0 + 3Cl20 → 2Al+3 Cl3-1 Intramolecular oxidation 2KCl+5O3-2
- 11. The value of redox reactions Redox reactions are very common. They linked the metabolic processes in
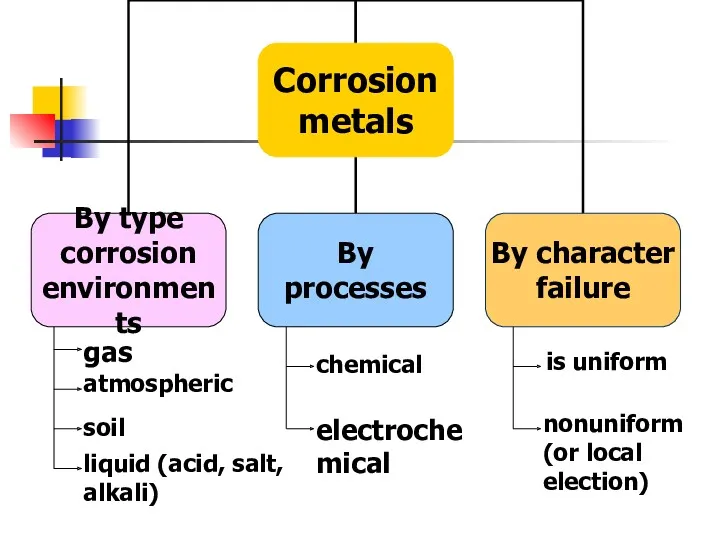
- 12. Corrosion of metals Methods corrosion protection
- 13. CORROSION - spontaneous destruction of metals and alloys as a result of chemical and electrochemical interactions
- 14. Factors that may cause corrosion Oxygen and atmospheric moisture Carbon and sulfur gases contained in the
- 15. gas atmospheric soil liquid (acid, salt, alkali) chemical electrochemical is uniform nonuniform (or local election)
- 16. CHEMICAL - a failure of metals and alloys as a result of their chemical interactions with
- 17. Electrochemical - a failure of metals, which is accompanied by the appearance of an electric current
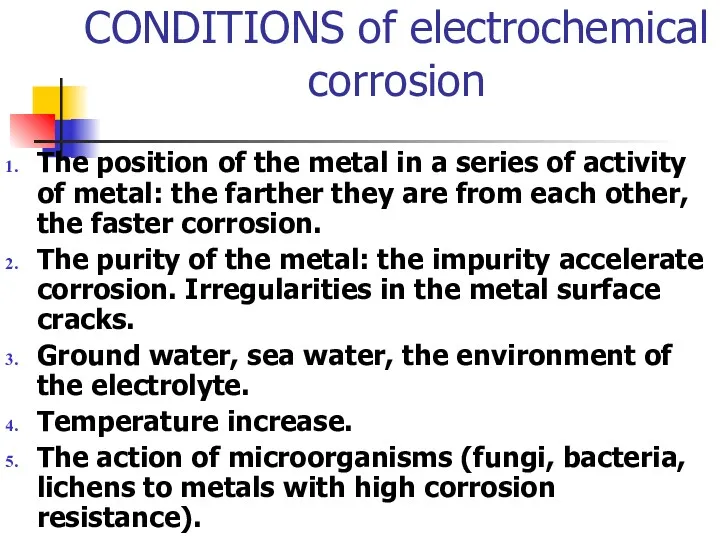
- 18. CONDITIONS of electrochemical corrosion The position of the metal in a series of activity of metal:
- 19. METHODS corrosion protection The application of protective coatings (paints, varnishes, enamels); Covering other metals (gold-plated, silver,
- 21. Скачать презентацию

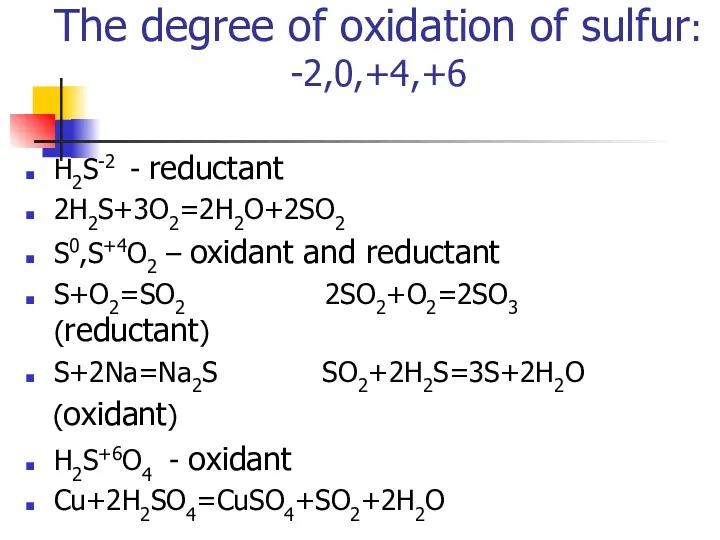







 Защитные материалы
Защитные материалы Органічна хімія, частина 1
Органічна хімія, частина 1 Методы восстановления и окисления
Методы восстановления и окисления Мыло. Его состав и моющее действие. Синтетические моющие средства
Мыло. Его состав и моющее действие. Синтетические моющие средства Химическая термодинамика. Термохимия
Химическая термодинамика. Термохимия Классификация и номенклатура органических соединений. (Лекция 1)
Классификация и номенклатура органических соединений. (Лекция 1) Кремний и его соединения
Кремний и его соединения Химиялық элементтердің тірі және өлі табиғатта таралуы
Химиялық элементтердің тірі және өлі табиғатта таралуы Кількість речовини. Моль - одиниця кількості речовини. Число Авогадро
Кількість речовини. Моль - одиниця кількості речовини. Число Авогадро Получение полимеров из низкомолекулярных соединений
Получение полимеров из низкомолекулярных соединений Характеристика химического элемента по его положению в периодической системе элементов Д.И.Менделеева
Характеристика химического элемента по его положению в периодической системе элементов Д.И.Менделеева Кислородсодержащие соединения серы. Оксиды, кислоты, соли
Кислородсодержащие соединения серы. Оксиды, кислоты, соли Классификация химических реакций
Классификация химических реакций Припекание взаимно растворимых твердых тел
Припекание взаимно растворимых твердых тел Химическая промышленность. Минеральные удобрения
Химическая промышленность. Минеральные удобрения Классификация химических реакций
Классификация химических реакций Качественные реакции на функциональные группы. Классификация функциональных групп
Качественные реакции на функциональные группы. Классификация функциональных групп Нефть. Углеводороды
Нефть. Углеводороды Спирты. Классификация
Спирты. Классификация Ненасыщенные (непредельные) углеводороды. Алкены (олефины)
Ненасыщенные (непредельные) углеводороды. Алкены (олефины) Тему Соли. Нитрат серебра(I) AgNO3
Тему Соли. Нитрат серебра(I) AgNO3 Витамины
Витамины Искусственная радиоактивность. Ядерное оружие и его поражающие факторы
Искусственная радиоактивность. Ядерное оружие и его поражающие факторы Sources of alkanes and cycloalkanes. Crude oil
Sources of alkanes and cycloalkanes. Crude oil Photocatalysts based on AgCl / Ag nanocomposites
Photocatalysts based on AgCl / Ag nanocomposites Синтетические топлива
Синтетические топлива Вещества и тела. Состояния веществ. Смеси
Вещества и тела. Состояния веществ. Смеси Модифицированные природные полимеры. Их свойства
Модифицированные природные полимеры. Их свойства