Слайд 21

Literature
1.Basic literature :
1. Jenkins, Chemistry, ISBN 978-0-17-628930-0
2. Alberta Learning, Chemistry
data booklet 2010, product №755115, ISBN 10645246
3.М.К.Оспанова, К.С.Аухадиева, Т.Г. Белоусова Химия: Учебник 1,2 часть для 10 класса естественно-математического направления общеобразовательных школ Алматы: Мектеп, 2019г.
4.М.К.Оспанова, К.С.Аухадиева, Т.Г. Белоусова Химия: Учебник 1,2 часть для 11 класса естественно-математического направления общеобразовательных школ Алматы: Мектеп, 2020 г.
5. М.Оспанова, К.Аухадиева, Т.Белоусова Химия. Дәрислик. 1, 2-қисим Алматы: Мектеп, 2019
6. М.Успанова, К.Аухадиева, Т. Белоусова Химия. Дарслик. 1, 2 - қисм Алматы: Мектеп, 2019
7. Т.Г.Белоусова, К.С. Аухадиева Химия: Методическое руководство 1, 2 часть естественно-математического направления общеобразовательных школ Алматы: Мектеп, 2019 г.
8. Темирбулатова А., Сагимбекова Н., Алимжанова С.,Химия. Сборник задач и упражнений Алматы: Мектеп, 2019 г.















 Figure
Figure Радиоактивные элементы почв
Радиоактивные элементы почв Янгишиева
Янгишиева Алкены (олефины). 10 профиль. Лекция №1
Алкены (олефины). 10 профиль. Лекция №1 Омыватель лобового стекла
Омыватель лобового стекла Хімічні властивості кислот
Хімічні властивості кислот Фенолы
Фенолы Хімічна рівновага. Принцип зміщення хімічної рівноваги
Хімічна рівновага. Принцип зміщення хімічної рівноваги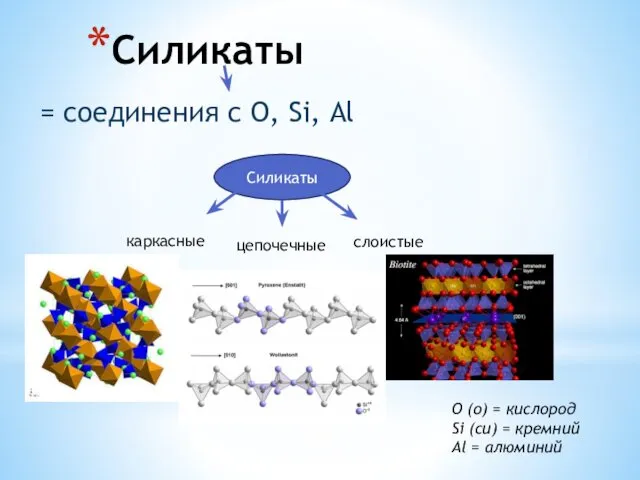 Силикаты. Слюды. Тальк. Фосфаты. Апатит. Крокоит
Силикаты. Слюды. Тальк. Фосфаты. Апатит. Крокоит Ароматические углеводороды. 10 класс
Ароматические углеводороды. 10 класс Электронное и пространственное строение молекул органических соединений – основа их биологической активности
Электронное и пространственное строение молекул органических соединений – основа их биологической активности Кристаллофизика_часть_1
Кристаллофизика_часть_1 Сплав золота и серебра - электрум
Сплав золота и серебра - электрум Растворы электролитов и неэлектролитов. Ионное произведение воды
Растворы электролитов и неэлектролитов. Ионное произведение воды Сірке қышқылын алу технологиясы
Сірке қышқылын алу технологиясы Кислоты. Яблочная кислота
Кислоты. Яблочная кислота Химиялық байланыс және заттардың құрылымдық түрлі сатылары
Химиялық байланыс және заттардың құрылымдық түрлі сатылары NaHSO4. Гидросульфат натрия
NaHSO4. Гидросульфат натрия Теория электролитической диссоциации
Теория электролитической диссоциации Уравнения химических реакций
Уравнения химических реакций Лекарства дома
Лекарства дома Таблица элементов
Таблица элементов Алкены – непредельные углеводороды. Получение, химические свойства и применение
Алкены – непредельные углеводороды. Получение, химические свойства и применение Химические свойства оксидов
Химические свойства оксидов Предмет и задачи фармацевтической химии. Общие методы анализа лекарственных средств неорганической природы согласно ГФУ
Предмет и задачи фармацевтической химии. Общие методы анализа лекарственных средств неорганической природы согласно ГФУ Жиры и масла в косметическом производстве
Жиры и масла в косметическом производстве Загальна характеристика неметалічних елементів. Неметали як прості речовини. Явище алотропії
Загальна характеристика неметалічних елементів. Неметали як прості речовини. Явище алотропії Кислородсодержащие соединения серы. Оксиды серы
Кислородсодержащие соединения серы. Оксиды серы